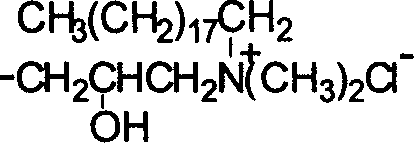Patents
Literature
151 results about "Ethylamine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ethylamine hydrochloride; ethylamine, 14C-labeled; from MeSH. Depositor-Supplied Synonyms. Chemical names and identifiers provided by individual data contributors and associated to PubChem Substance records. Synonyms of Substances corresponding to a PubChem Compound record are combined.
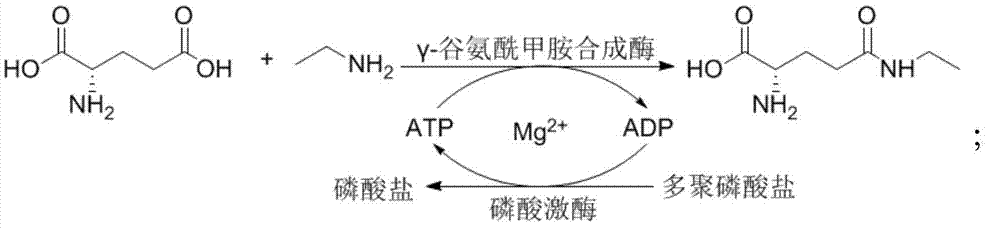
Method for synthesizing L-theanine through enzyme process
ActiveCN103409475AMake up for the shortcomings of poor stabilityHigh activityFermentationEscherichia coliChemical synthesis
The invention discloses a method for synthesizing L-theanine through an enzyme process, and belongs to the biotechnical field. The method is characterized in that a gamma-glutamyltranspeptidase gene is obtained through chemical synthesis, a gene engineering bacterium over-expressing gamma-glutamyltranspeptidase is constructed by treating Escherichia coli as a host bacterium, glutamine and ethylamine hydrochloride having different concentrations are acted by a recombinase, and theanine is efficiently produced at a temperature of 37-50DEG C under a pH value of 9.5-10.5. The enzyme source preparation process has the advantages of simplicity, low cost, and large enzyme amount, and the theanine production method has the advantages of simplicity, high conversion rate, high output, short time and the like, and is in favor of the industrialized amplification production.
Owner:JIANGNAN UNIV
Preparation method of lapatinib intermediate and analogues thereof
The invention discloses a compound preparation method, which comprises the following steps: a) a compound disclosed in formula I and 4-chlorine-6-iodine-quinazoline are in backflow reaction in isopropanol; b) kieselguhr, stannous chloride, trifluoroacetic acid, H2PdCl4 and polyvinylpyrrolidone are mixed in water and heated to 100-150 DEG C, and a Pd catalyst is obtained after reaction; c) the products obtained in step a), the Pd catalyst, 5-formyl furanboronic acid and K2CO3 are mixed to be reacted in a first organic solvent; and d) reaction products obtained in step c), organic base and 2-(methyl sulfone) ethylamine hydrochloride are mixed in a second organic solvent, and lapatinib basic groups or analogues thereof are obtained after reaction. The heterogeneous Pd catalyst is adopted, and because the heterogeneous Pd catalyst is easy to recycle, the preparation method provided by the invention protects the environment at the time of saving raw materials. Formula (I) is disclosed in the specification.
Owner:UNIV OF SCI & TECH OF CHINA
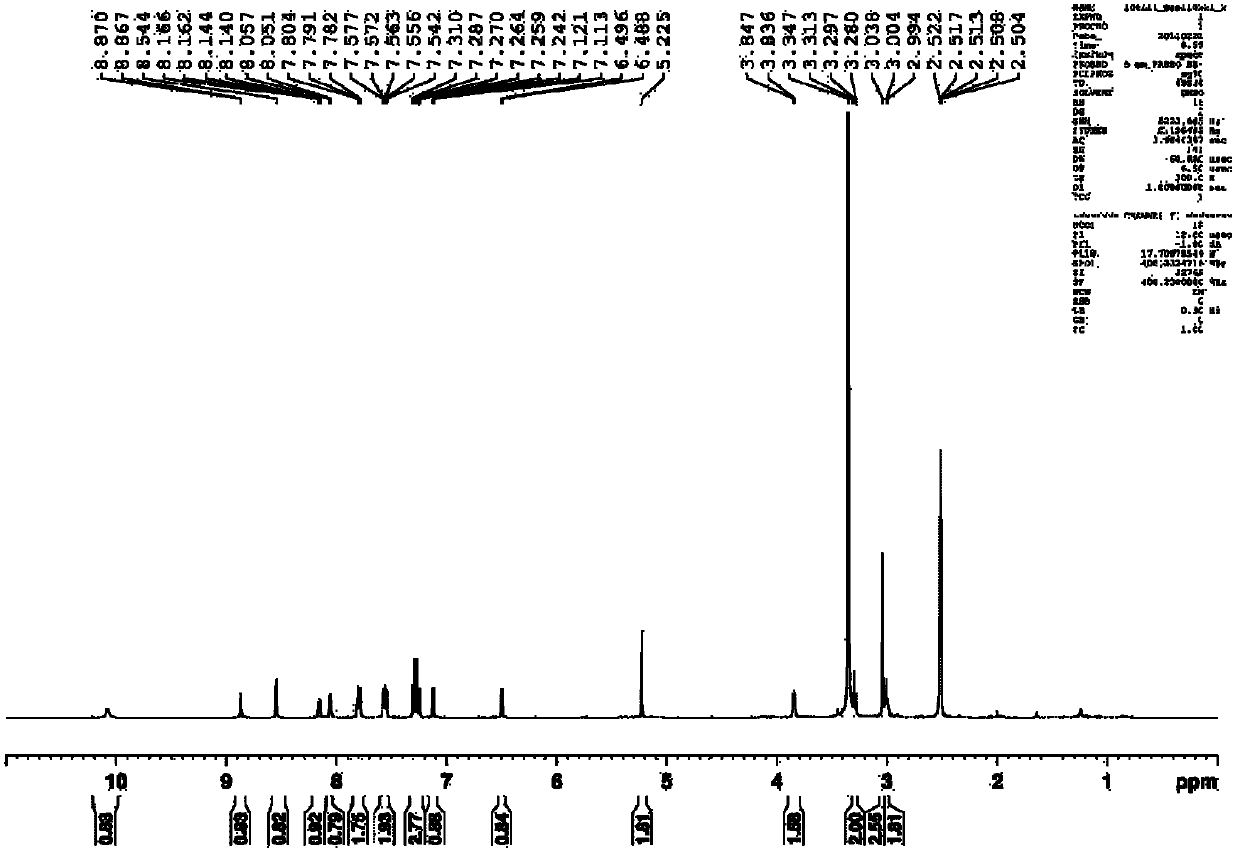
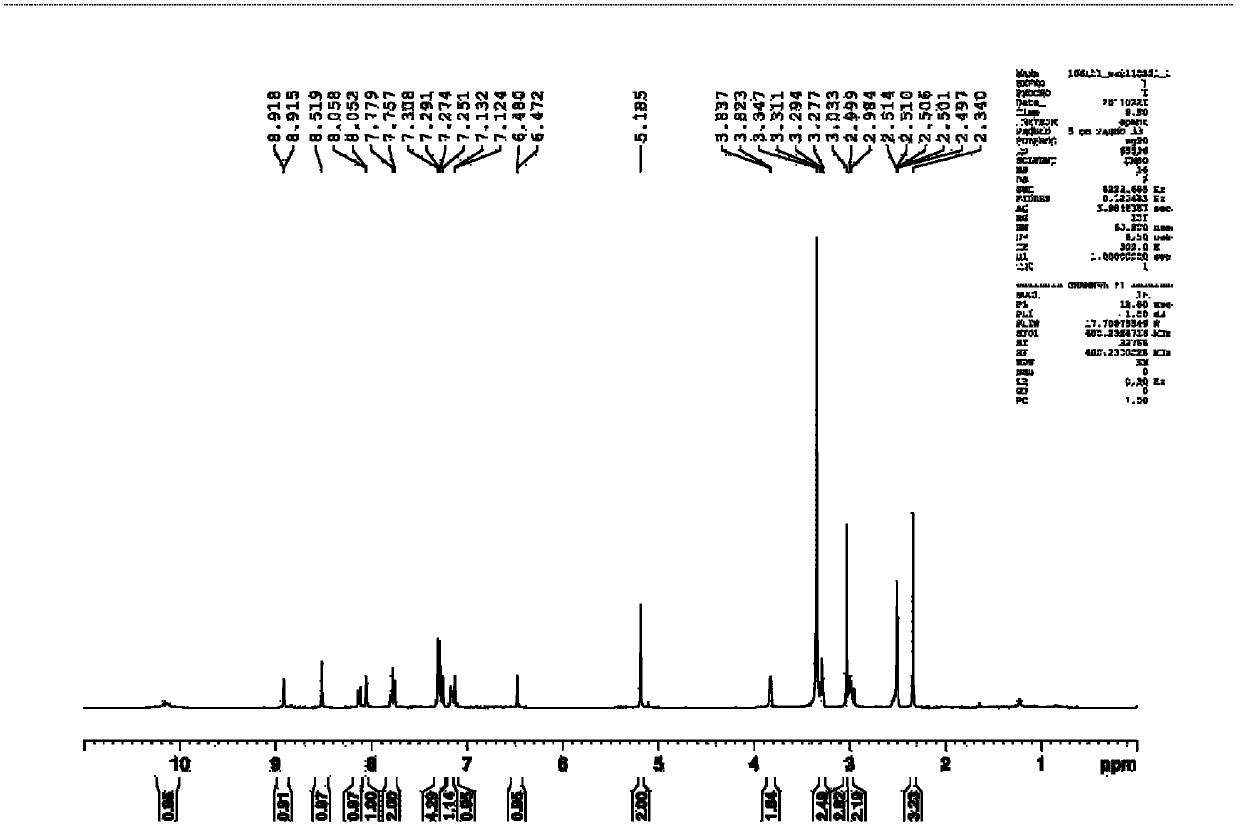
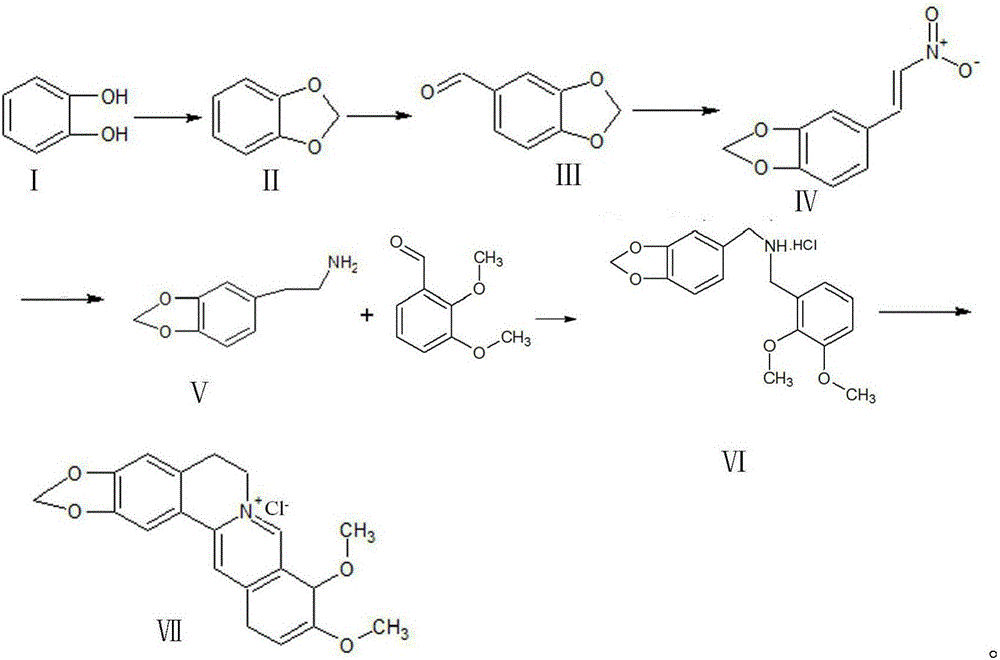
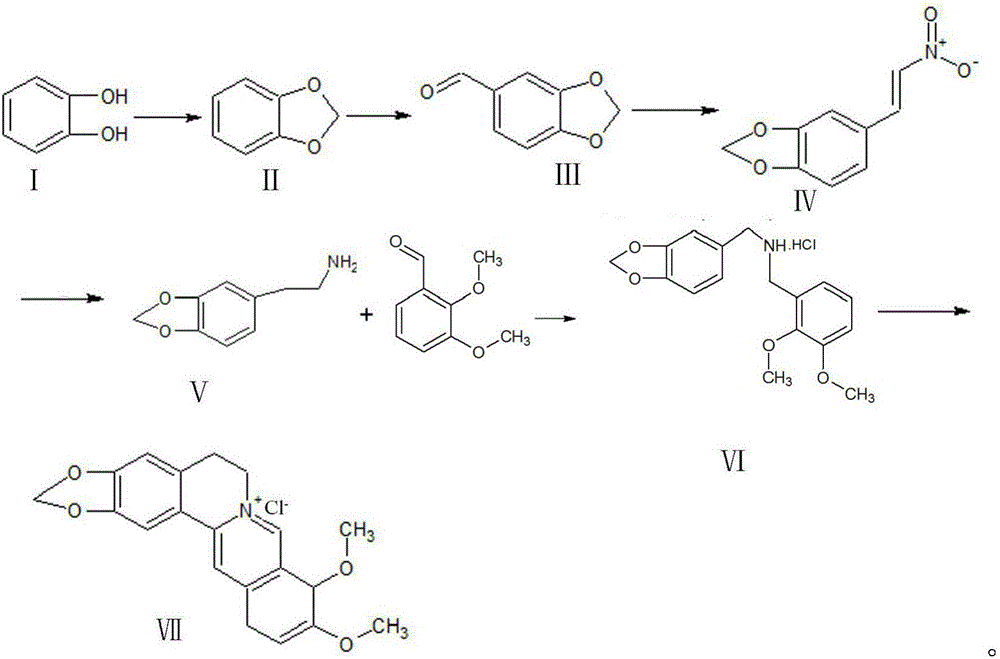
Synthetic process of berberine
The invention discloses a synthetic process of berberine. The synthetic process comprises the following synthetic route: pyrocatechol and dichloromethane are taken as raw materials and dimethyl sulphoxide is taken as a solvent to synthesize 1,3-benzodioxole; 1,3-benzodioxole is subjected to ViLsmeiar formylation to synthesize heliotropin; heliotropin undergoes nitration through a Henry reaction to generate beta-nitro-3,4-dioxomethenyl styrene; beta-nitro-3,4-dioxomethenyl styrene is subjected to Clemmensen reduction to generate 3,4-(methylenedioxyphenyl)ethylamine; and 3,4-(methylenedioxyphenyl)ethylamine and 2,3-dimethoxy benzaldehyde condense, and the obtained product is reduced to generate N-2,3-dimethoxybenzyl [3,4-(methylenedioxy)phenyl]ethylamine hydrochloride. N-2,3-dimethoxybenzyl [3,4-(methylenedioxy)phenyl]ethylamine hydrochloride is cyclized in a condition of glyoxal, formic acid and copper sulphate to generate berberine hydrochloride. According to the synthetic route, cyanation is avoided and toxicity is reduced. Zinc amalgam is adopted to replace H2Ni and LiAlH4, so that the cost is greatly reduced, the process difficulty is reduced, and the product yield is increased.
Owner:佑华制药(乐山)有限公司
Synthetic method of L-theanine
The invention relates to the technical field of bioengineering and in particular relates to a synthetic method of L-theanine. The method comprises the following step of synthesizing L-theanine by taking gamma-glutamyl-methylamine synthetase and phosphokinase as catalysts and L-sodium glutamate and ethylamine hydrochloride as substrates. The method can be used for producing a lot of cloned expression proteins as catalysts by taking L-sodium glutamate and ethylamine hydrochloride which are relatively low in price as the initial raw materials, and has the advantages of being mild in reaction condition, strong in specificity, high in reaction conversion rate, simple and convenient to operate, low in requirement on reaction equipment, small in environmental pollution and the like.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Xanthiphenyl ketamine or its salt and its preparing process
A process for preparing xanthiphenyl ketoamine or its salt includes such steps as reflux reaction of p-hydroxy benzylidenacetone, N-methyl piperethanamine hydrochloride and polyformaldehyde in absolute alcohol for 6-9.5 hr until fully solidifying, filtering, washing, recrystallizing 1-2 times, then drying to obtain xanthiphenyl ketoamine hydrochloride, neutralizing to obtain xanthiphenyl ketoamine, and reaction on acid to obtain the corresponding salt. Its advantages are simple process, high output rate and high purity.
Owner:WUXI JIMIN KEXIN SHANHE PHARMA
Method for selective synthesis of alpha-narcotine with participation of blockage group
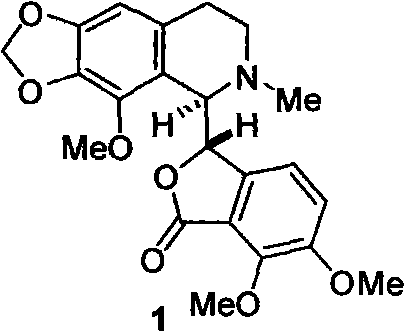
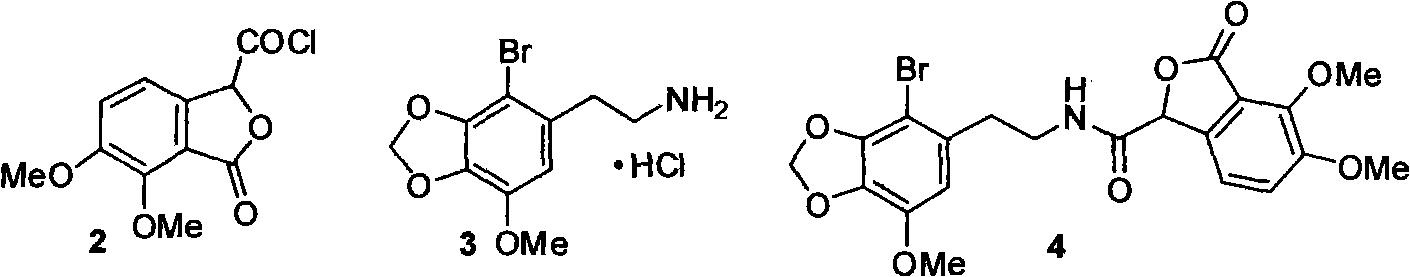
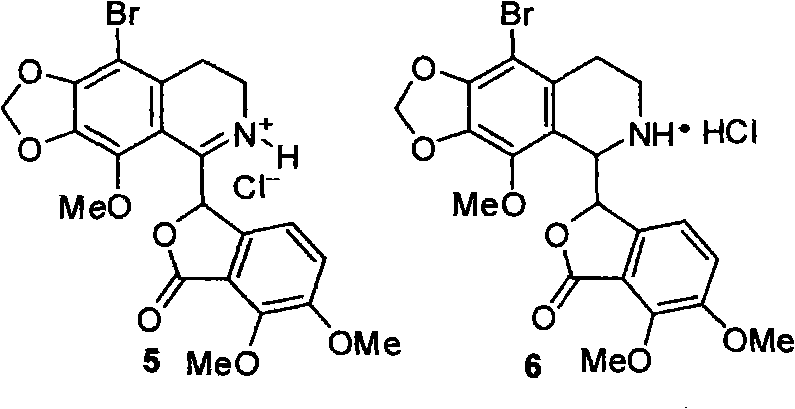
The invention belongs to the technical field of drug synthesis, and relates to position selective synthesis of alpha-narcotine with participation of a blockage group, preparation of serial phthalide tetrahydroisoquinoline compounds and preparation of various phthalide-3-carboxylic acid compounds and various 3-oxo isochroman-1-carboxylic acid compounds through a universal mode. In the method, 2, 3-dimethoxy benzoic acid is used as a raw material and condensed with glyoxylate through the universal mode to obtain 6, 7-dimethoxy phthalide-3-carboxylic acid, the carboxylic acid and 2-(2-bromo-3, 4-methylenedioxy-5-methoxy-phenyl)-ethylamine hydrochloride are condensed to prepare amide, the amide reacts with Bischler-Napieralski to prepare bromo-alpha-narcotine through reaction, methylation and salt formation, and (-)-alpha-narcotine is then prepared through dehalogenation and resolution. Due to the positioning function of the blockage group, the invention effectively reduces the position selectivity of Bischler-Napieralski cyclization, and realizes the simple and efficient synthesis of alpha-narcotine through removal of the blockage group and the chiral resolution.
Owner:SHENYANG PHARMA UNIVERSITY
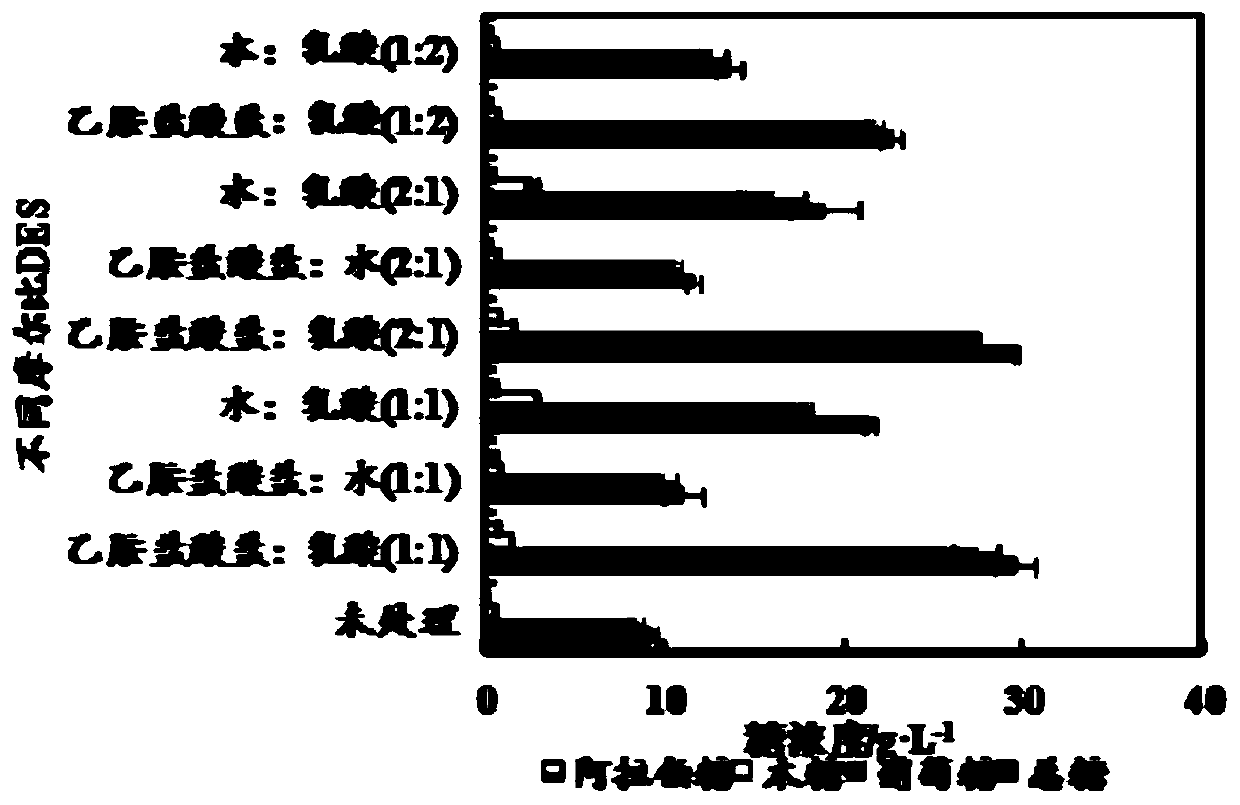
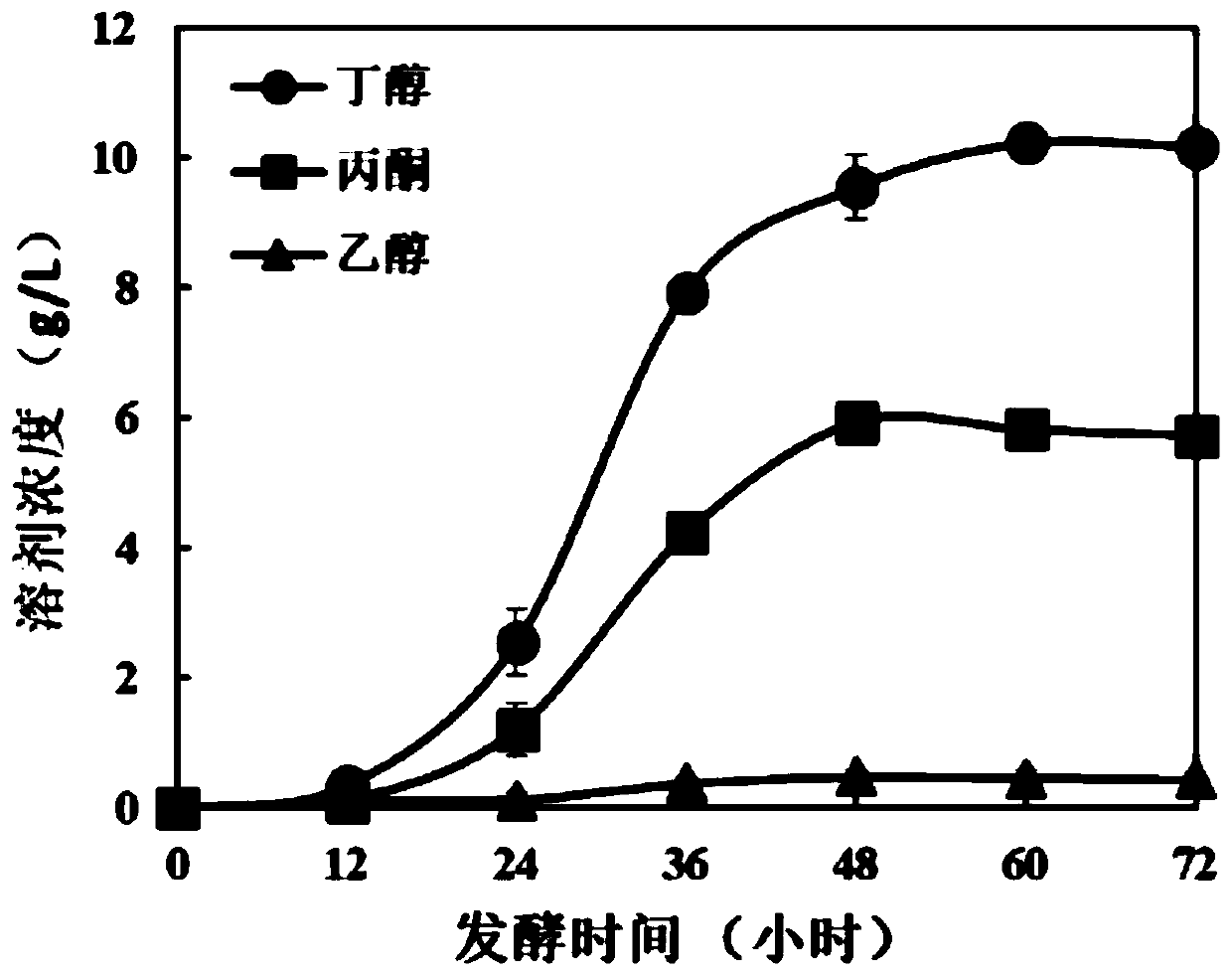
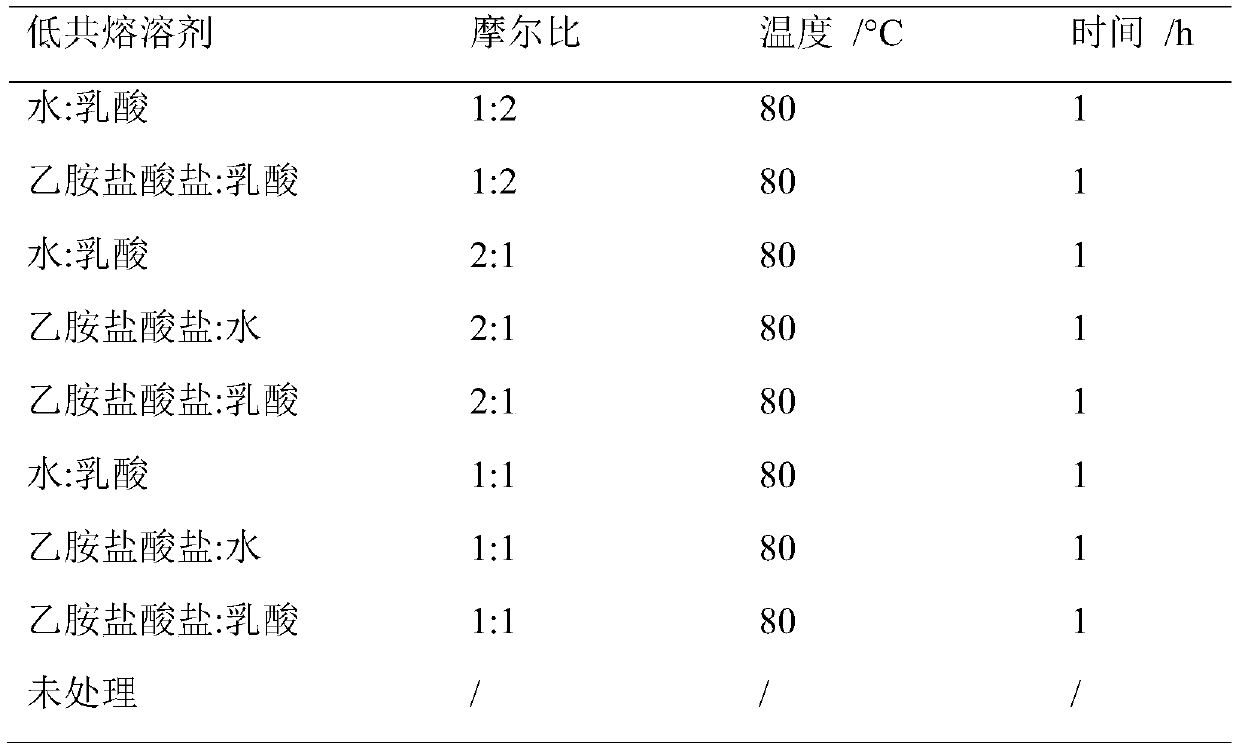
Method for fermenting butanol by performing pretreatment on straw by adopting novel eutectic solvent
InactiveCN109852639ALow costThree wastes lessMicroorganism based processesFermentationConcentrations glucoseSolvent
The invention discloses a method for fermenting butanol by performing pretreatment on straw by adopting a novel eutectic solvent, and belongs to the technical field of bioengineering. According to themethod for fermenting the butanol by performing pretreatment on the straw by adopting the novel eutectic solvent, pretreatment is performed on straw by using a novel donor eutectic solvent which is synthesized by using lactic acid as a hydrogen bond donor and dthylamine hydrochloride as a hydrogen bond receptor; enzymatic hydrolysis is carried out by adding cellulase so as to obtain an enzymatichydrolyzate; and then, the enzymatic hydrolyzate is utilized as a carbon source for fermenting the butanol. During the process of performing the pretreatment on the rice straw, relatively high hemicellulose removal rate (73.2%) and lignin removal rate (55.7%) are achieved; and total sugar concentration after enzymatic hydrolysis can be up to 59 g / L, wherein the glucose concentration is 58.3 g.L<-1>. Compared with an existing method for fermenting the butanol by adopting a chloride choline : formic acid : acetic acid ionic-liquid (42 g / L), the method has relatively great improvement on the total sugar concentration. The whole processes have the advantages of being low in cost, few in waste gas, waste water and industrial residues and so on. In addition, the hydrolyzate of the straw after the pretreatment can be used for producing the butanol by performing fermentation.
Owner:JIANGNAN UNIV
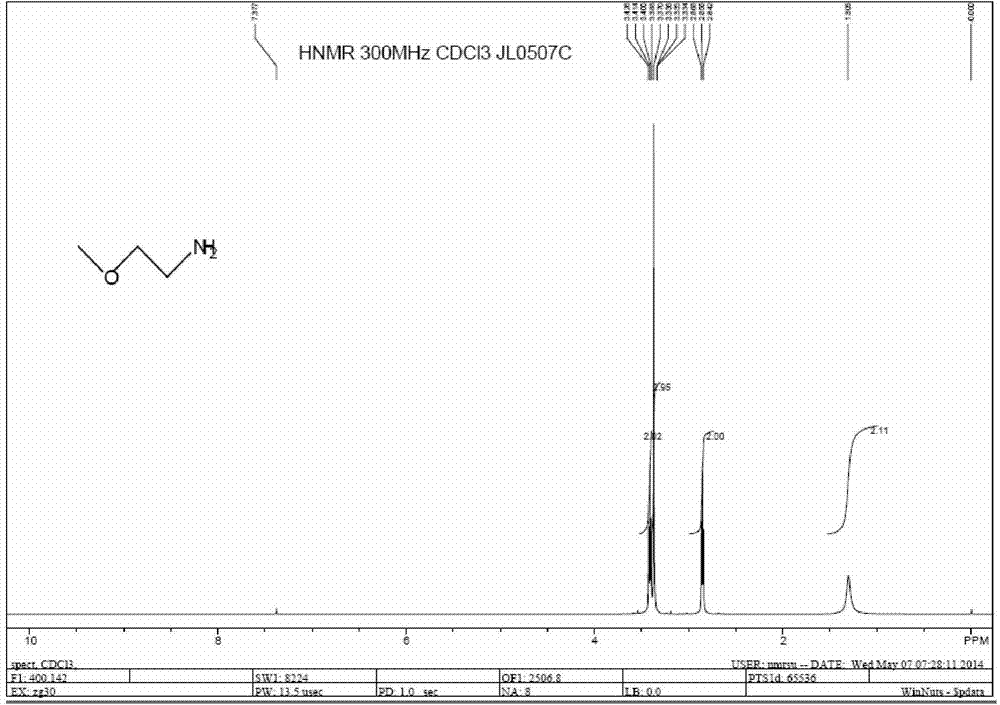
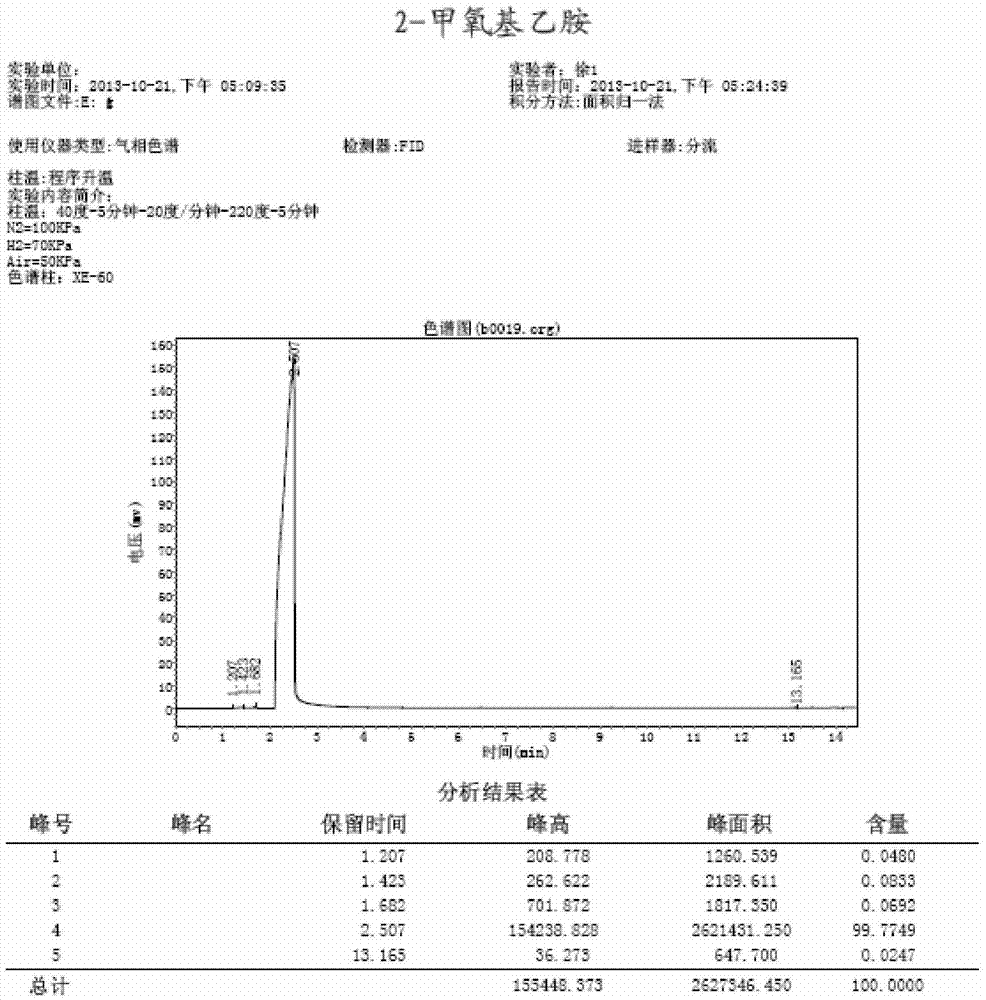
Preparation method of 2-methoxy ethylamine
InactiveCN103936599AHigh yieldLow costOrganic compound preparationAmino-hyroxy compound preparationBenzaldehydeAldimine
The invention relates to a preparation method of 2-methoxy ethylamine. The preparation method comprises the following steps: preparing a benzyl imine intermediate; preparing an N-benzyl alkenyl-2-methoxy ethylamine intermediate; preparing a 2-methoxy ethylamine hydrochloride aqueous solution; preparing a 2-methoxy ethylamine solution; and desolventizing the 2-methoxy ethylamine solution and rectifying, and collecting fractions at 82-85 DEG C, wherein the total yield is 56-84%, the purity is greater than 99.7% and the water content is less than 0.2%. According to the preparation method provided by the invention, by using cheap ethanol amine as a raw material, the final product 2-methoxy ethylamine can be obtained by the following steps: carrying out azeotropic dehydration with benzaldehyde to generate aldimine; then, methylating under an alkaline condition; and removing protection and alkalizing and rectifying. The preparation method is low in production cost, fewer in three wastes, safe, easy to operate and suitable for industrialization.
Owner:SHANGHAI TBBMED CO LTD
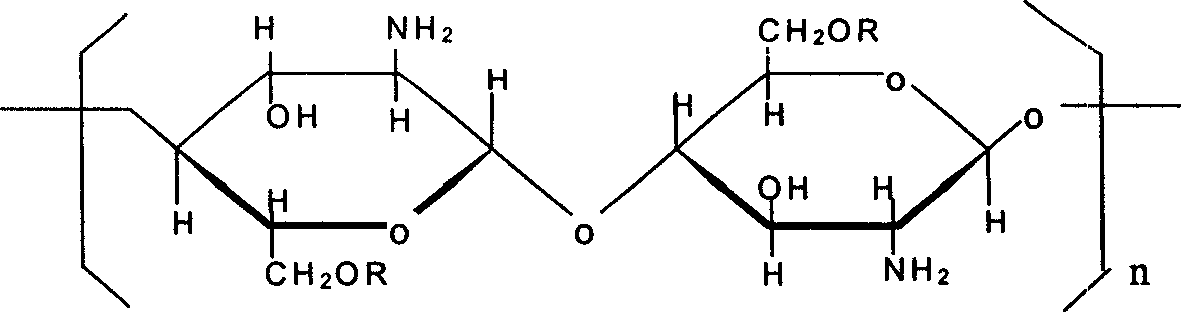
Water and oil soluble O-chitosan derivatives and their preparation and use
InactiveCN1594365AImprove adsorption capacityObvious weight loss effectOrganic active ingredientsMetabolism disorderEpichlorohydrinHydrochloride
Water and oil soluble O-chitosan derivatives, their preparation and use thereof, wherein the synthesized chitosan upper substitutive derivative comprises o-ethlamine hydroxide ethyl chitosan and 0-2' -hydroxylpropyl -N, N-dimethyl octadecyl ammonium chloride chitosan, which is prepared through the reaction of N-phthaloyl chitosan with chlorohydrin, chlorethamin hydrochlorate and N,N dimethyl octadecyl amine hydrochlorate and epichlorohydrin in dimethyl carbinol medium.
Owner:TIANJIN UNIV
Leuprorelin synthesis method
InactiveCN105330726AHigh yieldHigh purityLuteinising hormone-releasing hormonePeptide preparation methodsDipeptideLeuprorelin
The invention discloses a leuprorelin synthesis method. According to the method, a dipeptide midbody is obtained through liquid-phase synthesis and then used for solid-phase synthesis to generate full-protection nonapeptide peptide resin, full-protection nonapeptide is cut off from the resin and then inoculated with ethylamine in the liquid phase to generate full-protection leuprorelin, and a leuprorelin crude product is obtained through splitting decomposition. The method specifically comprises the steps of obtaining Fmoc-Leu-OSU fat through condensation under the action of a condensing agent; reacting with H-Arg(pbf)-OH under the action of alkali to generate Fmoc-Leu-Arg(pbf)-OH; obtaining Fmoc-Pro-CTC Resin through reaction under the action of the alkali; removing Fmoc, and conducting amino acid coupling in sequence under the action of a condensing agent to generate full-protection nonapeptide peptide resin; cutting the full-protection nonapeptide peptide resin with a cutting reagent to generate full-protection nonapeptide; generating leuprorelin full-protection peptide by means of full-protection nonapeptide and ethylamine hydrochloride under the action of a condensing agent, and obtaining the leuprorelin crude product through splitting decomposition. By the adoption of the method, the yield and purity of leuprorelin are improved remarkably, ammonolysis is not needed, and reaction is easy and controllable. The method is suitable for industrial production.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
One-step synthesis of ethyl isocyanate
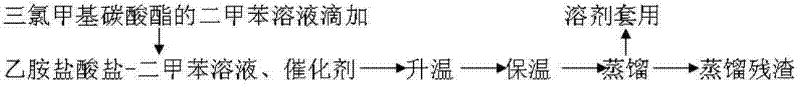
ActiveCN102659631AIsocyanic acid derivatives preparationOrganic compound preparationXylylenePtru catalyst
The invention relates to one-step synthesis of ethyl isocyanate, which belongs to the chemical technical field. The method abandons a process of phosgene feeding in routine techniques, and uses xylene as a solvent.150-200 kg ethylamine hydrochloride is dissolved in 400-600 L xylene; the xylene solution containing 200-300 kg trichloromethyl carbonate is added into a reaction vessel in a dropwise manner with the presence of 5-10 kg of a catalyst; and the following steps are carried out: preparation of a catalyst, synthesis of a target product, separation of the target product, synthesis of ethyl isocyanate, and preparation of the product after separation. The method in the invention abandons the process of phosgene feeding in the routine techniques, and uses xylene as the solvent. The method effectively solves the problems of high risk, severe pollution, high cost and poor quality and the like in the process of phosgene feeding in the productive process in the routine techniques that use phosgene.
Owner:平原倍斯特化工有限公司
Recovering and applying method of triethylamine in acylation liquid for preparing levofloxacin 1
ActiveCN102153479AEasy to operateNo distillationOrganic compound preparationAmino compound preparationAlkalinityOrganic solvent
The invention relates to a recovering and applying method of triethylamine in an acylation liquid for preparing levofloxacin 1 by a 2,3,4,5-tetrachloro benzoyl chloride acxylation method, belonging to the technical field of fine chemicals. The method is realized by the following steps: 1) filtering the acylation liquid in the process of preparing the levofloxacin by using the 2,3,4,5-tetrachloro benzoyl chloride acxylation method, so as to separate out the triethylamine, wherein the filtrate is an acylated mother liquid, and a filter cake is triethylamine hydrochloride; and 2) adding liquid alkaline to regulate the filter cake triethylamine hydrochloride to alkalinity, so that the triethylamine hydrochloride is converted into the triethylamine hydrochloride to be separated out, extractingwith an organic solvent, dehydrating and converting for synthesis. The triethylamine recovering method is simple to operate, rectification is not needed, and the recovery rate is high and reaches above 93% which is equivalent to the fact that about 35 kg of triethylamine is just added in production of 1 ton of levofloxacin 1, and for expensive triethylamine, production cost is greatly reduced.
Owner:ZHEJIANG LANGHUA PHARMA
Production process for synthetizing L-theanine through biological immobilized enzyme catalysis
InactiveCN102533886ASpecific process responseHigh yieldOn/in organic carrierFermentationSODIUM METAPHOSPHATEMonosodium glutamate
The invention relates to a production process for synthetizing L-theanine through biological immobilized enzyme catalysis, which belongs to the technical field of biosynthesis. The L-theanine is synthetized through the biological immobilized enzyme catalysis by taking sodium glutamate, magnesium chloride hexahydrate, sodium hexametaphosphate, ethylamine hydrochloride and triphosadenine to serve as raw materials. The production process for synthetizing the L-theanine through the biological immobilized enzyme catalysis comprises the following process steps: (1) performing catalytic synthesis on a substrate and an immobilized enzyme to generate the L-theanine, and then, centrifugally separating the biological immobilized enzyme from reaction liquid; (2) removing anions and cations in the reaction liquid through cation exchange resin to generate purified L-theanine; (3) decoloring an L-theanine aqueous solution through activated carbon, and performing vacuum film concentrating to obtain an L-theanine concentrated solution; and (4) adding 95 percent of alcohol to the L-theanine concentrated solution to separate out white L-theanine precipitation crystals; centrifugally separating; and performing vacuum drying to obtain white L-theanine powder. The production process for synthetizing the L-theanine through the biological immobilized enzyme catalysis has the advantages of specific process reaction, high yield, high purity, short period, low energy consumption and the like and is suitable for industrial production.
Owner:广东乐尔康生物科技股份有限公司
Treatment process for glyphosate mother liquid
InactiveCN101508700AHigh yieldReduce consumptionGroup 5/15 element organic compoundsWater/sewage treatmentPhosphateAqueous solution
The invention discloses a treatment process of glyphosate mother solution. The glyphosate mother solution is the remaining mother solution obtained after separating glyphosate bulk drug from a system when an acidolysis reaction is over during a process of producing the glyphosate bulk drug by a dialkyl phosphate method. The treatment process of the glyphosate mother solution comprises the following steps: containing the glyphosate mother solution in a concentration device; separating to eliminate most of water under a depressurization condition to separate the glyphosate bulk drug dissolved in the mother solution; standing for layering, wherein, an upper layer is triethylamine hydrochloride and a lower layer is aqueous solution containing the glyphosate; combining the solution of the lower layer with the glyphosate for the concentration treatment process of next batch; neutralizing the triethylamine hydrochloride of the upper layer with alkali to adjust pH value to 8-13; rectifying the triethylamine of the upper layer and returning to a glyphosate synthesis procedure for recycle. The solution of the lower layer is distilled to recover a small amount of the triethylamine hydrochloride, and then discharged after biochemical treatment or used for preparing 10% glyphosate solution. The treatment process is suitable for treatment of the glyphosate mother solution.
Owner:陈桢铭 +1
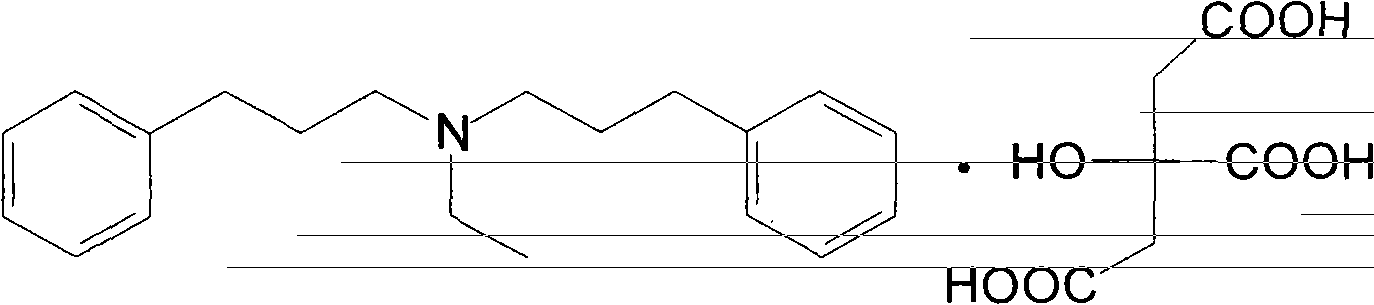
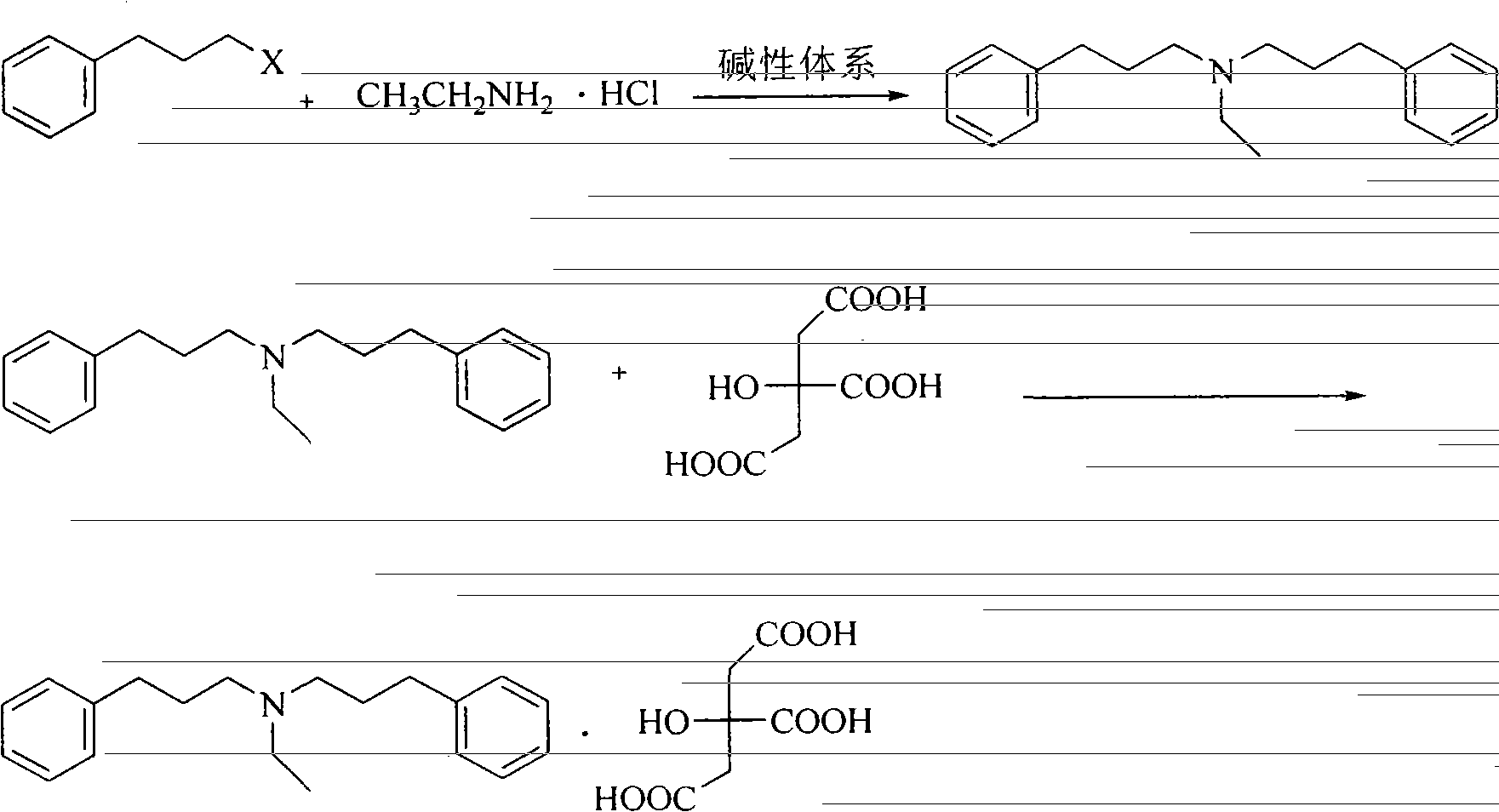
Novel preparation method of alverine citrate
ActiveCN103508898AEasy to operateHigh yieldAmino preparation from aminesOrganic layerAlverine citrate
The invention discloses a novel preparation method of alverine citrate, which comprises the following steps: reacting 3-phenylhalopropane and ethylamine hydrochloride used as initial raw materials in an alkaline system to generate diphenylpropyl ethylamine, and refining under the interaction between the organic layer and the citric acid to obtain the alverine citrate. The process route has the characteristics of accessible raw materials, fewer reaction steps, mild conditions, high yield and the like and is simple to operate, and thus, is an environment-friendly synthesis route which can easily implement industrialization.
Owner:河北凯盛医药科技有限公司
2-arylsulfonyl-2, 2-difluorodiazoethane compound, preparation method and application thereof
InactiveCN108383761AEasy to manufactureEasy to useOrganic chemistryOrganic compound preparationCycloadditionAlkyne
The invention relates to a 2-arylsulfonyl-2, 2-difluorodiazoethane compound, a preparation method and application thereof. The preparation method of the 2-arylsulfonyl-2, 2-difluorodiazoethane compound includes: dissolving 2, 2-difluoro-2-aryl sulfonyl ethylamine hydrochloride in a mixed solvent of an organic solvent and water, adding nitrite, carrying out stirring at room temperature for completereaction, and then conducting washing, extraction, separation and column chromatography purification to obtain the yellow liquid 2-arylsulfonyl-2, 2-difluorodiazoethane. The obtained target compoundis a diazo compound containing sulfonyl difluoromethyl, can be subjected to [3+2] cycloaddition reaction with alkyne so as to synthesize a pyrazole compound containing difluoromethyl. The difluorodiazoethane compound provided by the invention has the characteristics of convenient preparation, simple use and mild reaction conditions, and the invention provides an effective method for synthesis of compounds containing difluoromethyl.
Owner:TIANJIN UNIV
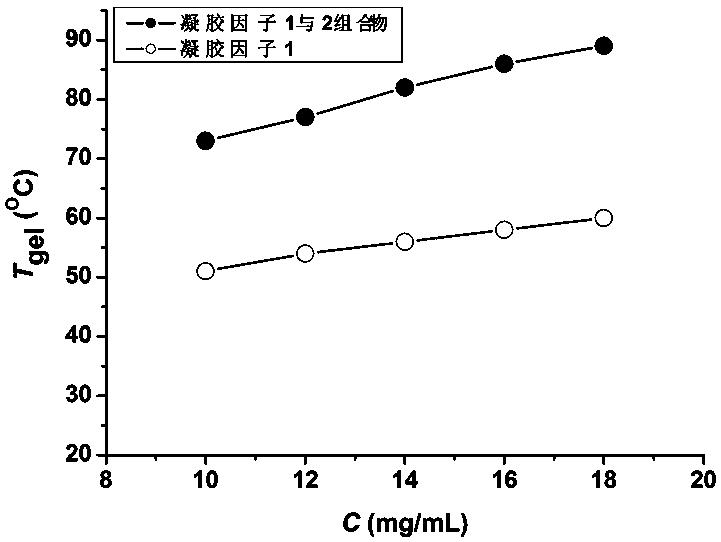
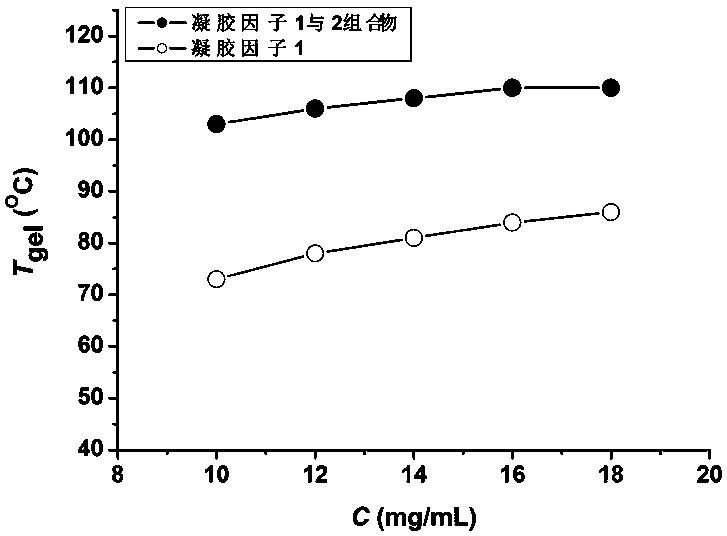
High-thermal stability two-component organic gel and preparation method thereof
ActiveCN107551962AImprove thermal stabilityHigh gel-solution phase transition temperatureColloidal chemistry detailsOrganic solventThermal stability
The invention discloses a high-thermal stability two-component organic gel. The high-thermal stability two-component organic gel is a mixed system which is prepared from N<1>,N<3>,N<5>-tris(2-ethylhexyl)phenyl-1,3,5-triformamide serving as a first gel factor, triethylamine hydrochloride serving as a second gel factor and a low-polarity organic solvent. According to the two-component organic gel disclosed by the invention, a porous three-dimensional reticular super-molecular structure is constructed through a synergistic effect between the first gel factor and the second gel factor; the formedorganic gel has high thermal stability, namely, gel-solution phase transition.
Owner:ZHONGBEI UNIV
Theanine synthetase gene and method for preparing theanine
InactiveCN106119214ARefining steps are simpleLess side effectsLigasesFermentationEscherichia coliPhase filter
The invention discloses a theanine synthetase gene and a method for preparing theanine. The total length of a synthetase gene cDNA is 2,886 bp, the length of a reading frame is 2,532 bp, and a gene cDNA sequence is as shown in SEQ ID No.1. The theanine synthetase gene can be used for synthesizing and preparing theanine. The method particularly comprises the steps that 1, a target gene, as shown in SEQ ID No.1, of a nucleotide sequence is obtained; 2, pMAL-c5x servers as a vector, the target gene is connected to the vector, a recombinant vector is obtained, and escherichia coli is led into the recombinant vector; 3, the escherichia coli led into the recombinant vector is subjected to induced expression, induced protein is produced, substrates of sodium glutamate and ethylamine hydrochloride are added, cultivation is conducted continuously for 12 h, products in a culture medium are collected and then subjected to centrifugation, supernatant is taken, filtration is conducted with an aqueous phase filter membrane of 0.22 micrometers, and a synthesized theanine solution is obtained. Due to the fact that the synthesizing method is similar to a synthetic reaction taking place in a plant body, the synthesizing method has the advantages of being few in side reaction, simple in product refining step and the like.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Preparation method of 2-[[[5-(dimethylamino)methyl-2-furanyl]methyl]thio]ethylamine
The invention provides a preparation method of 2-[[[5-(dimethylamino)methyl-2-furanyl]methyl]thio]ethylamine. The preparation method comprises mixing a dimethylamine hydrochloride solution, polyformaldehyde and a quaternary ammonium salt, heating the mixture to 50 to 70 DEG C, adding furfuryl alcohol into the reaction system, carrying out a first condensation reaction process to obtain an intermediate, mixing the intermediate, cysteamine hydrochloride, concentrated hydrochloric acid and perchloric acid, carrying out a second condensation reaction process at the system temperature of 15 to 25 DEG C to obtain a mixed solution of 2-[[[5-(dimethylamino)methyl-2-furanyl]methyl]thio]ethylamine hydrochloride, alkalifying the mixed solution, extracting the solution through dichloromethane and distilling the extract to obtain 2-[[[5-(dimethylamino)methyl-2-furanyl]methyl]thio]ethylamine. The preparation method has the advantages of high yield, high product purity, simple processes and industrialization easiness.
Owner:HEBEI HAILI FRAGRANCES CO LTD
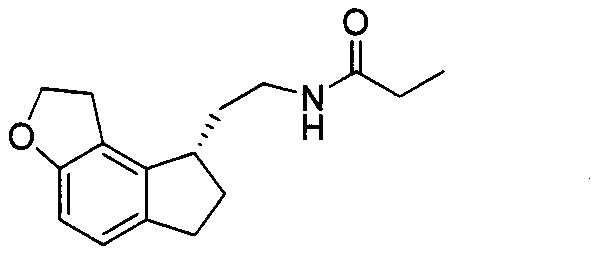
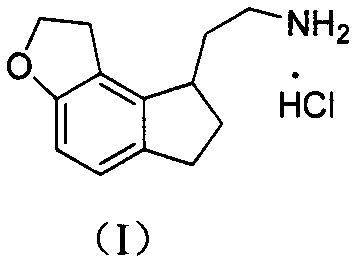
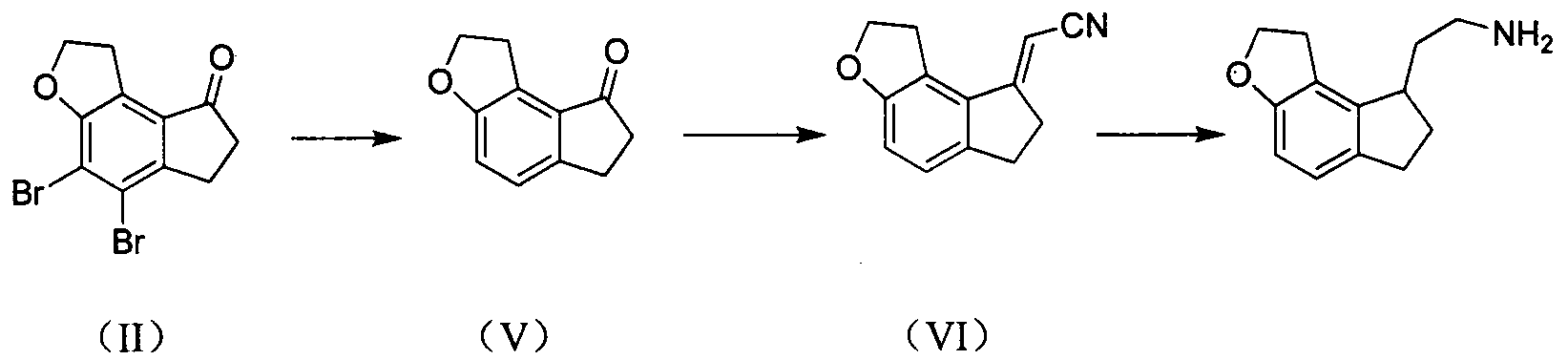
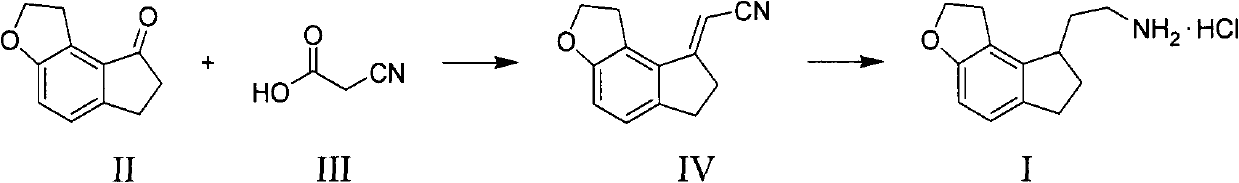
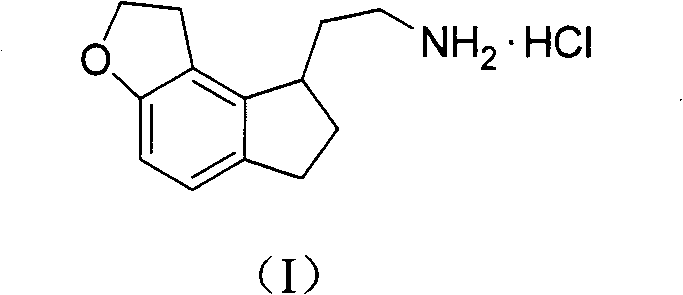
Preparation method of ramelteon intermediate
The invention discloses a novel preparation method of a ramelteon intermediate 2-(1, 2, 6, 7-tetrahydro-8H-indeno[5, 4-b] furan-8-yl) ethylamine hydrochloride (I). The method is characterized by comprising the following steps: dehydrating and condensing and heating and decarboxylating 4, 5-dibromo-1, 2, 6, 7-tetrahydro-8H-indeno[5, 4-b] furan-8-one (II) and cyanoacetic acid (III) under the effect of a catalyst to obtain a compound (IV); then, hydrogenating the (IV) and salifying to obtain the ramelteon intermediate hydrochloride (I). The invention provides a novel preparation of the ramelteon intermediate hydrochloride (I). According to the method, cyanoacetic acid (II) which is cheap and easily available is used, so that the method is simplified in synthetic step, high in efficiency and simple and convenient to operate, and the method is a preparation method which is more suitable for industrialized production.
Owner:CHINA PHARM UNIV
Tea tree water planting nutrient solution and preparation method thereof
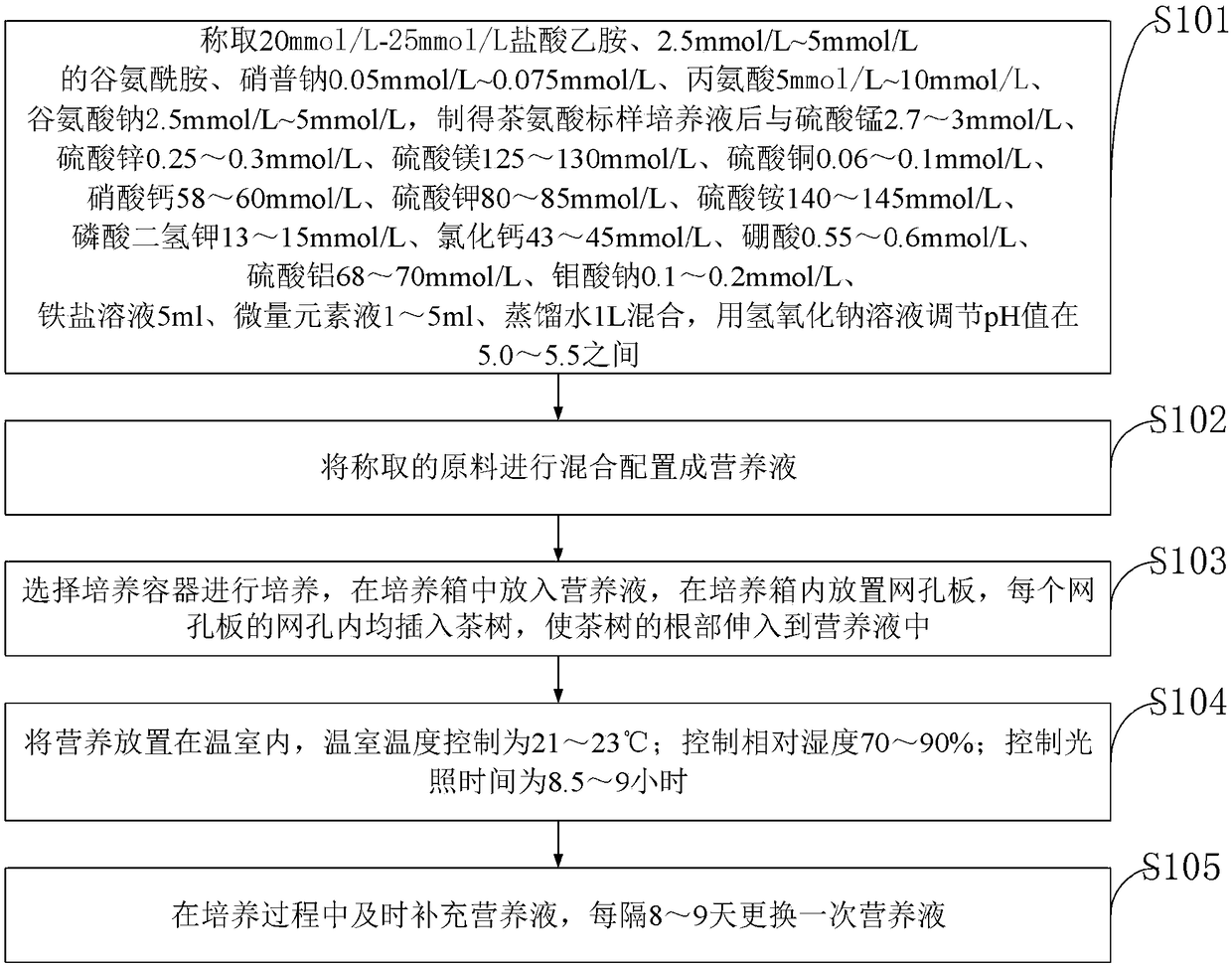
InactiveCN108530170AReasonable formulaGrow fastCalcareous fertilisersMagnesium fertilisersMonopotassium phosphateCopper sulfate
The invention belongs to the technical field of plant culture and discloses a tea tree water planting nutrient solution and a preparation method thereof. The preparation method of the tea tree water planting nutrient solution comprises, in 1L of distilled water, adding, by molar weight, ethylamine hydrochloride, glutamine, sodium nitroprusside, alanine, sodium glutamate, manganese sulfate, zinc sulfate, magnesium sulfate, copper sulfate, calcium nitrate, potassium sulfate, ammonium sulfate, monopotassium phosphate, calcium chloride, boric acid, aluminum sulfate, sodium molybdate, ferric salt solution and trace element solution. The tea tree water planting nutrient solution achieves good culturing effects and high plant resistance, and meanwhile, is reasonable in composition, enriches nutrients required by tea tree, ensures that saplings can acquire nutrition and further provides reliable preconditions for culture of the saplings; during culturing, temperature, humidity and light conditions are reasonably controlled, so that the tea trees can grow up rapidly, and further the yield as well as the economic benefits can be increased.
Owner:TEA INST YUNNAN ACADEMY OF AGRI SCI
Method for producing L-theanine by supplementing material
InactiveCN104372046AImprove conversion rateLow costMicroorganism based processesFermentationHigh concentrationL-theanine
The invention relates to a method for producing L-theanine by supplementing a material, belonging to the technical field of biology and solving the technical problems of enhancing the transformation rate of L-theanine in the presence of a high-concentration substrate and reducing the production cost by providing a process for supplementing the material at constant speed and supplementing the material at variable speed. The technical scheme is as follows: the method comprises the following steps of: producing L-theanine by adopting gamma-GGTase derived from a bacillus subtilis source; preparing L-glutamine and ethylamine hydrochloride according to a proportion of 1:5 to 1:15; predetermining temperature at 30-45 DEG C, regulating the pH value to be 9.0-11.0, then adding a 2-20 unit amount of gamma-GGTase to each milliliter of reaction liquid, supplementing a certain quantity of substrates every 2 hours, and reacting for 10-20 hours to obtain L-theanine with higher concentration.
Owner:JIANGNAN UNIV
Novel method for preparing ramelteon key intermediate
The invention discloses a novel method for preparing a ramelteon key intermediate 2-(1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-yl)ethylamine hydrochloride (I). The method is characterized by comprising the following steps of: performing dehydration condensation and heated decarboxylation on 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (II) and cyanoacetic acid (III) under the action of a catalyst to obtain a compound (IV); and directly hydrogenating and salifying the compound (IV) without separating and purifying to obtain the ramelteon key intermediate 2-(1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-yl)ethylamine hydrochloride (I). The invention provides the novel method for preparing the ramelteon key intermediate hydrochloride (I). In the method, the cheap and readily available cyanoacetic acid (II) is used; and the method is high in yield and low in cost, is easy and convenient to operate, is environment-friendly and is suitable for industrialized production.
Owner:CHINA PHARM UNIV
Tetracaine preparation method
ActiveCN105646261AHigh yieldThe synthesis steps are simpleOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidHydrochloride
The invention discloses a tetracaine preparation method, comprising the following steps in sequence: (1) performing a catalytic reduction reaction on para aminobenzoic acid and n-butanal to prepare N-butyl para aminobenzoic acid; (2) filtering reaction liquid obtained in step (1), then adding sodium hydroxide for mixing; (3) enabling the N-butyl para aminobenzoic acid treated by the sodium hydroxide to directly react with N,N-dimethyl chloroethylamine hydrochloride without refining and separation, thus obtaining tetracaine. According to the method, the steps are fewer, the operation is simple, more convenience and cleanness are realized, and the product yield is higher.
Owner:JINAN CHENGHUI SHUANGDA CHEM
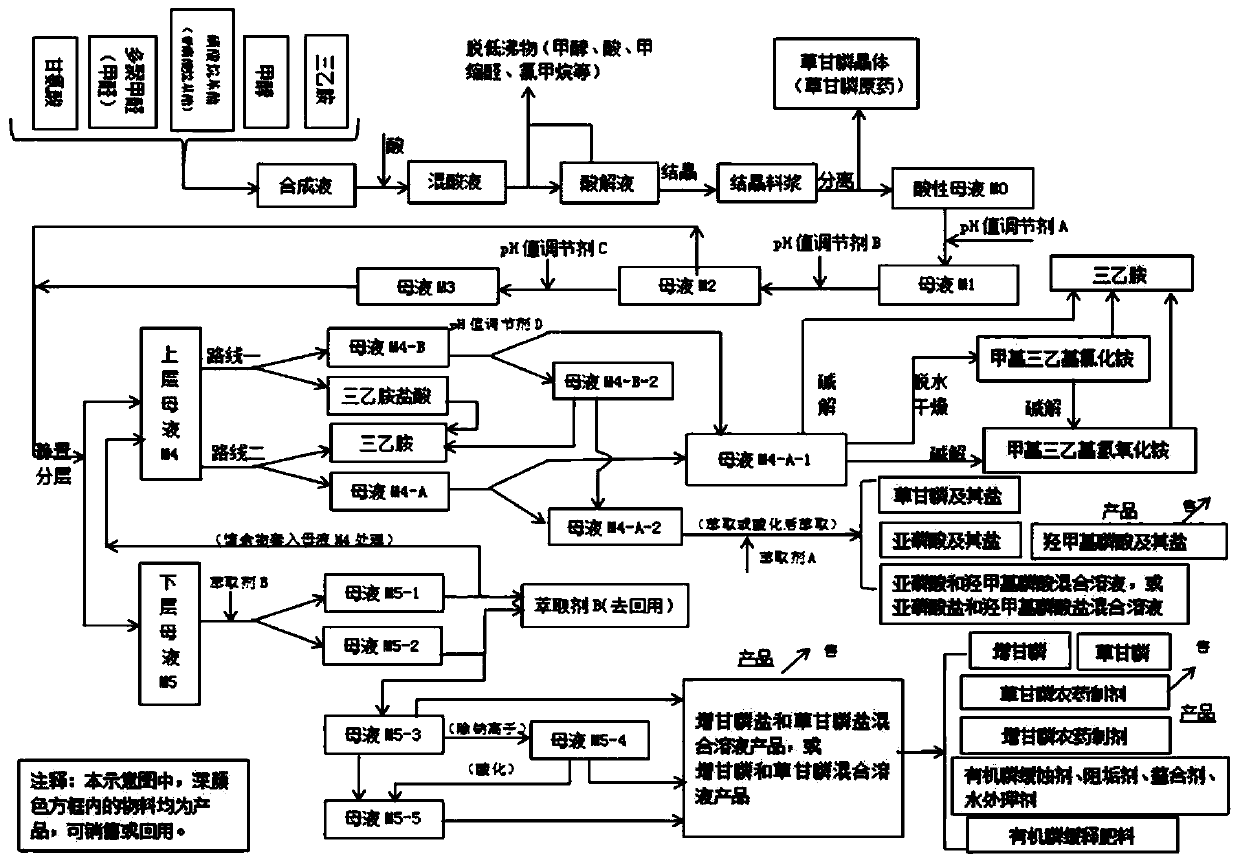
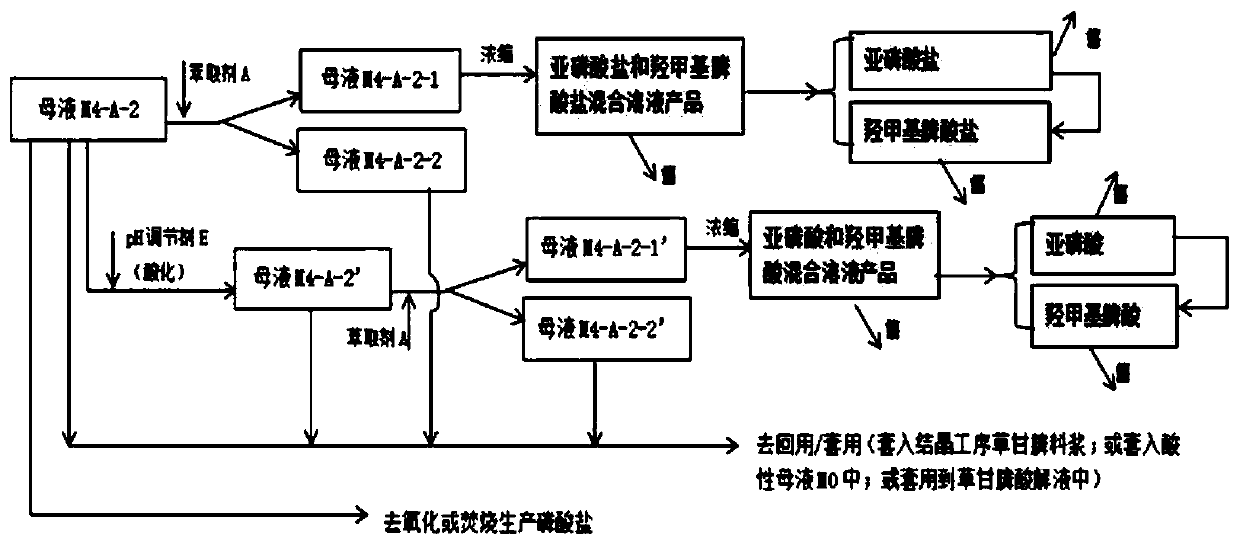
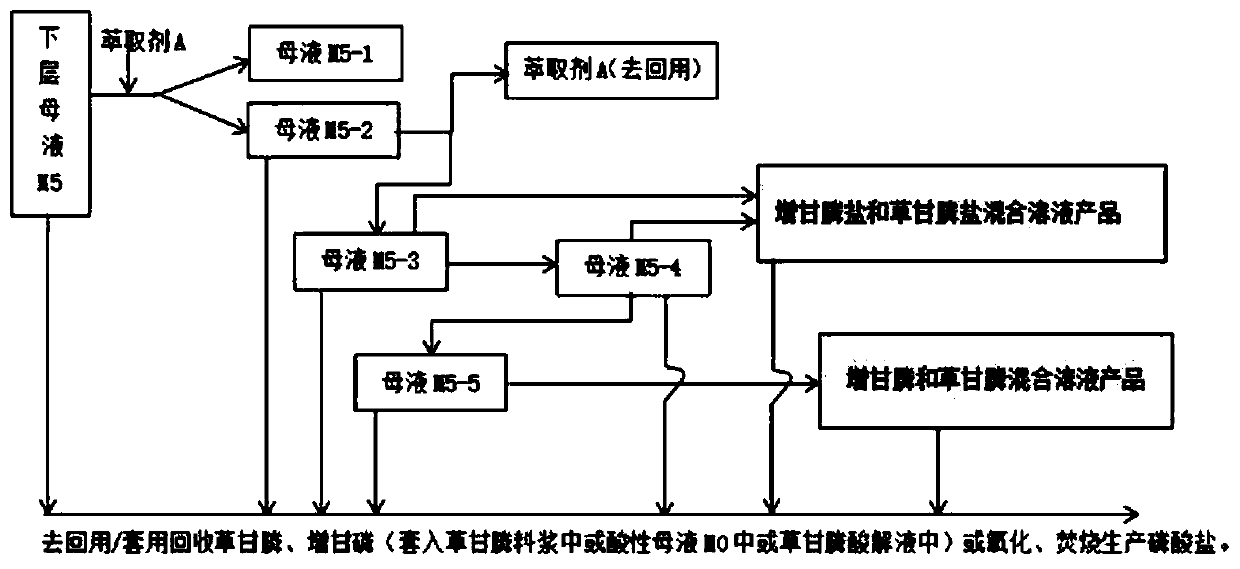
Glyphosate mother liquor comprehensive treatment and resource recycling method
PendingCN111333519AReduce volume and handling costsLess impuritiesAmino compound purification/separationGroup 5/15 element organic compoundsPhosphorous acidPulp and paper industry
The invention discloses a glyphosate mother liquor comprehensive treatment and resource recycling method which comprises the following steps: adding a pH value regulator into acidic mother liquor M0,standing for layering, and separating the solution to obtain mother liquor M4 at the upper layer and mother liquor M5 at the lower layer; the mother liquor M4 and the mother liquor M5 are treated andapplied, triethylamine hydrochloride, triethylamine, chloride salt, methyltriethylammonium chloride, phosphorous acid or salt thereof, hydroxymethylphosphonic acid or salt thereof, glyphosate or saltthereof, glyphosate or salt thereof and glyphosate or salt thereof are recovered from the mother liquor M4 and the mother liquor M5, and are respectively and correspondingly converted into products with higher additional values for utilization. The glyphosate mother liquor comprehensive treatment and resource recycling method has the advantages that the emission is reduced from the source; the method reduces the total amount and treatment load of the glyphosate mother liquor, reduces environmental pollution, realizes reasonable recycling and appreciation of resources, improves economic benefits, is environment-friendly, outstanding in economic benefits and good in technical implementation effect, and is suitable for large-scale industrial application.
Owner:陈兴华
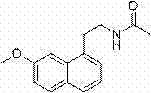
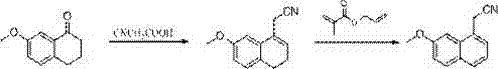
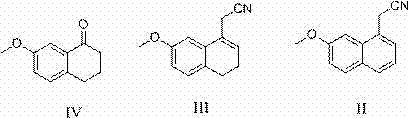
Synthetic method of 2-(7-methoxyl-1-naphthyl) ethylamine hydrochloride
InactiveCN103113243ASimple stepsHigh reaction yieldOrganic compound preparationAmino-hyroxy compound preparationCyanoacetic acidDehydrogenation
The invention relates to a synthetic method of 2-(7-methoxyl-1-naphthyl) ethylamine hydrochloride. The method comprises the following steps of: reacting 7-methoxyl-1-naphthyl (a compound IV) with cyanoacetic acid to prepare (7-methoxyl-3,4-dihydro-1-naphthyl) acetonitrile (a compound III); performing deep drawing quality (DDQ) dehydrogenation to prepare (7-methoxyl-1-naphthyl) acetonitrile; using sodium borohydride as a reducing agent and reacting sodium borohydride with calcium chloride in anhydrous tetrahydrofuran to react to obtain 2-(7-methoxyl-1-naphthyl) ethylamine; and satisfying with hydrochloric acid to obtain a compound I, wherein the compound is an important intermediate of synthetic agomelatine.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
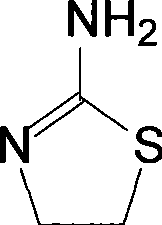
Method for synthesizing 2-amino thizaoline
ActiveCN101417985AFew reaction stepsAdvanced process routeOrganic chemistry2-aminothiazolineOrganic solvent
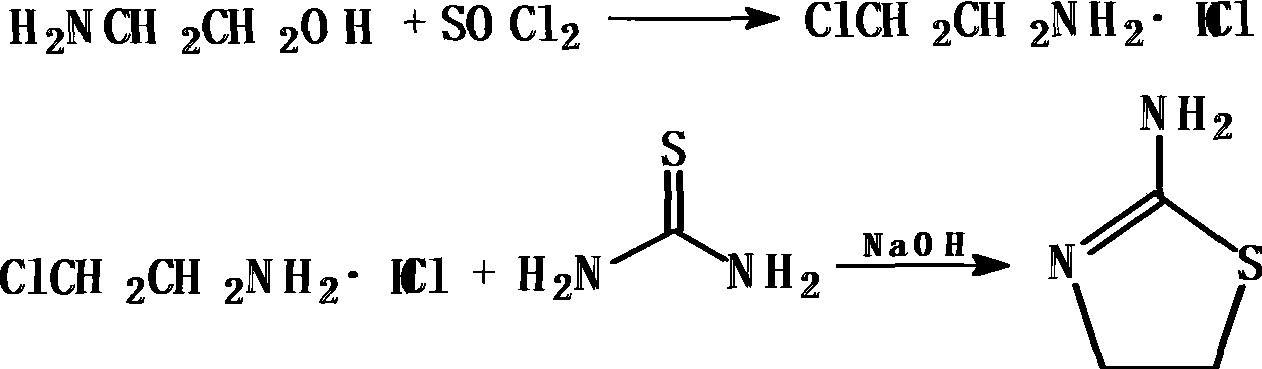
The invention relates to a method for synthesizing 2-aminothiazoline, which comprises the following steps: firstly, performing chlorinated reaction between ethanolamine and thionyl chloride in an organic solvent to generate 2-chloro-ethylamine hydrochloride; and secondly, performing cyclization reaction between the 2-chloro-ethylamine hydrochloride and thiourea to generate the 2-aminothiazoline. Compared with the prior art, the method does not need introduce hydrogen chloride gas and concentrated hydrochloric acid to synthesize ethanolamine hydrochloride, thereby reducing reaction reagents and the reaction steps; besides, the process route is more advanced, environment-friendly and safer, and the cyclization yield reaches more than 70 percent.
Owner:山西新天源药业有限公司
Clean production process of glycine and co-produced ammonium chloride
InactiveCN106699591AAmino compound purification/separationOrganic compound preparationGlycineHydrogen
The invention provides a clean production process of glycine. The clean production process can be used for co-producing an ammonium chloride product when being used for producing glycine. In a process of producing glycine, a triethylamine hydrochloride product can be co-produced. After a solvent, namely methanol, and a catalyst are added into a reactor of a glycine ammonolysis reaction, solid ammonium chloroacetate is added; after the temperature is raised to 65 DEG C, triethylamine is added; all triethylamine is added within one and a half hours; and the heat is kept and a reaction is carried out for two and a half hours until the pH (Potential of Hydrogen) value is 7.5. The glycine and triethylamine hydrochloride are separated through cooling and freezing respectively. The triethylamine in the triethylamine hydrochloride is recycled through an ammonia introducing method; and meanwhile, the ammonium chloride product is co-produced.
Owner:QINGDAO SENMEIKE CHEM TECH CO LTD
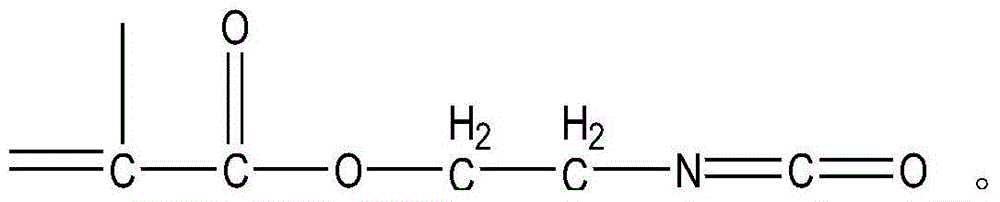
Preparation method for isocyanate ethyl methacrylate
InactiveCN104086458ANo pollution in the processEasy to separateIsocyanic acid derivatives preparationOrganic compound preparationMethacrylateAlcohol
The invention discloses a preparation method for isocyanate ethyl methacrylate. The preparation method comprises the following steps: (I), reacting by adopting ethanol amine, methacrylic acid and an organic solvent as raw materials in the presence of thionyl chloride, adding distilled water after reaction is ended, stirring, layering, concentrating and drying an obtained water layer to obtain methylacryloyl(2-hydroxyl) ethylamine hydrochloride, and drying and concentrating the organic phase to obtain methacrylic acid (2-hydroxyl) alcohol ester; (II), adding an organic solvent, solid phosgene and a catalyst into the reactor, adding methylacryloyl(2-hydroxyl) ethylamine hydrochloride generated in the step (I), heating to 80-100 DEG C in a reaction process, and collecting the fraction which is isocyanate ethyl methacrylate after the reaction is ended. The preparation method disclosed by the invention serves double purposes, and waste gas generated in the reaction process is absorbed by alkali liquor, so that environmental pollution is almost avoided; the purity of obtained products is over 95%, and the product yield is over 90%.
Owner:ZHANGJIAGANG HICOMER CHEM CO LTD
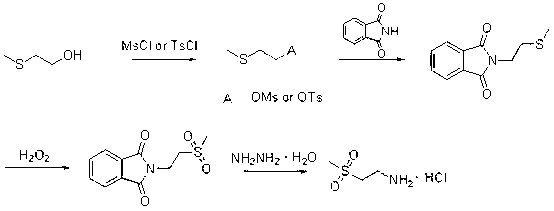
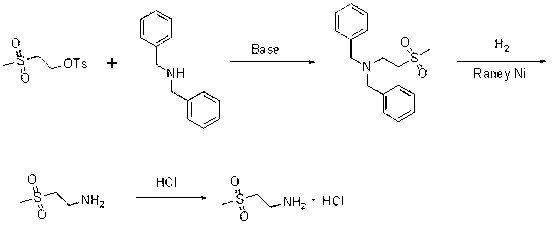
Preparation method of lapatinib intermediate
InactiveCN103030580AOrganic chemistryOrganic compound preparationMethylthioethanolMedicinal chemistry
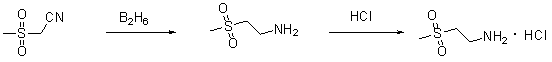
The invention provides a preparation method of a lapatinib intermediate 2-methylsulfonylethylamine hydrochloride. The invention is characterized in that 2-methylthioethanol used as an initial raw material is subjected to esterification, SN2 substitution, oxidation and hydrazinolysis to obtain the target product. The method provided by the invention is beneficial to lowering the environmental pollution, is simple to operate, has the advantages of mild reaction conditions, high product purity and cheap and accessible raw material, and is suitable for industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com