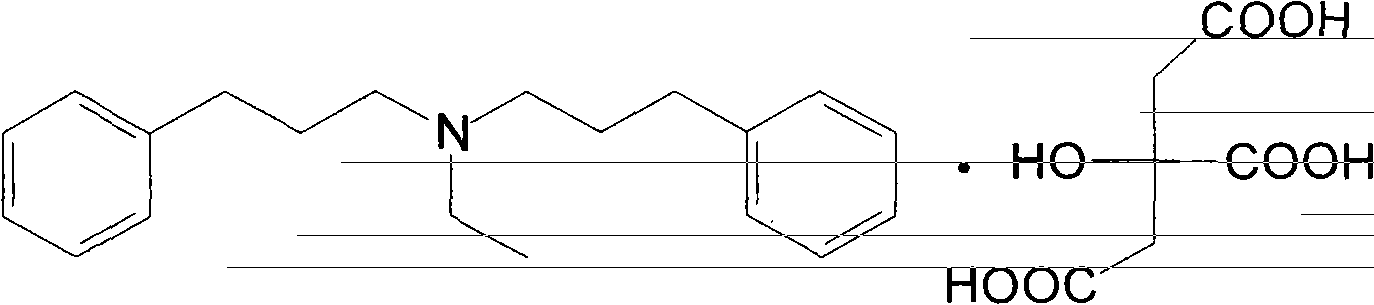
Novel preparation method of alverine citrate
A technology of alverine citrate and ethylamine hydrochloride, which is applied in the field of preparation of alverine citrate, can solve the problems of inconvenient use and storage, difficult to realize, long steps, etc., and achieves reduction of risks and simplification of processes Mild effect of process, reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
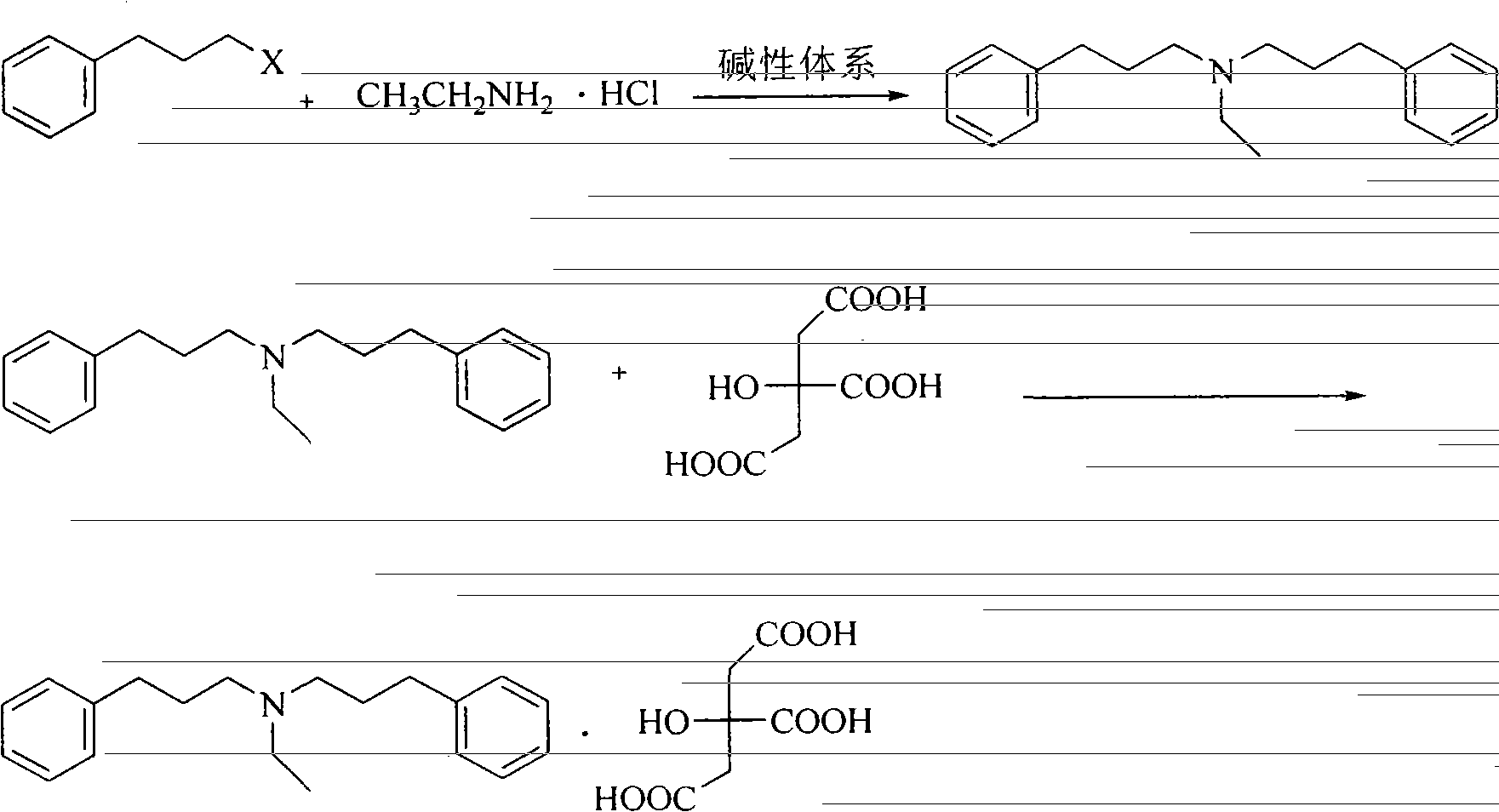
[0013] Embodiment 1: the preparation of alverine citrate
[0014] The first step: the preparation of diphenylpropylethylamine
[0015] Add 20 g (0.10 mol) of 3-phenylbromopropane, 4.1 g (0.05 mol) of ethylamine hydrochloride, 0.2 g of tetrabutylammonium bromide and 50 ml of sodium hydroxide solution (35%-40%) into a 250 ml flask. Stir and heat up to 85-90°C, keep warm for reaction, and track with TLC (ethyl acetate / petroleum ether=2:1). After the reaction, the aqueous layer was extracted three times with ethyl acetate, and the organic layers were combined. Add anhydrous sodium sulfate to dry. After filtration, the filtrate was concentrated under reduced pressure to obtain 13.5 g of brown-yellow liquid. Yield: 96%.
[0016] The second step: the preparation of alverine citrate
[0017] Dissolve 13.5 g of the above liquid in 100 ml of absolute ethanol, stir to dissolve, then add 9.2 g (0.048 mol) of citric acid into a solution of 100 ml of absolute ethanol, stir at room temp...
Embodiment 2
[0018] Embodiment 2: the preparation of alverine citrate
[0019] The first step: the preparation of diphenylpropylethylamine
[0020] Add 15.5g (0.10mol) of 3-phenylchloropropane, 4.1g (0.05mol) of ethylamine hydrochloride, 0.2g of tetrabutylammonium bromide and 50ml of sodium hydroxide solution (35%-40%) into a 250ml flask. Stir and heat up to 85-90°C, keep the reaction warm, and follow up with TLC. After the reaction, the aqueous layer was extracted three times with ethyl acetate, and the organic layers were combined. Add anhydrous sodium sulfate to dry. After filtration, the filtrate was concentrated under reduced pressure to obtain 12.4 g of brown-yellow liquid. Yield: 88%.
[0021] The second step: the preparation of alverine citrate
[0022] Dissolve 12.4 g of the above liquid in 90 ml of absolute ethanol, stir to dissolve, then add 8.5 g (0.044 mol) of citric acid into a solution of 90 ml of absolute ethanol, stir at room temperature to precipitate a solid. Filte...
Embodiment 3
[0023] Embodiment 3: the preparation of alverine citrate
[0024] The first step: the preparation of diphenylpropylethylamine
[0025] Add 20 g (0.10 mol) of 3-phenylbromopropane, 4.1 g (0.05 mol) of ethylamine hydrochloride and 50 ml of sodium hydroxide solution (35%-40%) into a 250 ml flask. Stir and heat up to 85-90°C, keep the reaction warm, and follow up with TLC. After the reaction, the aqueous layer was extracted three times with ethyl acetate, and the organic layers were combined. Add anhydrous sodium sulfate to dry. After filtration, the filtrate was concentrated under reduced pressure to obtain 10.8 g of brown-yellow liquid. Yield: 76%.
[0026] The second step: the preparation of alverine citrate
[0027] Dissolve 10.8 g of the above liquid in 80 ml of absolute ethanol, stir to dissolve, then add 7.4 g (0.039 mol) of citric acid into a solution of 80 ml of absolute ethanol, stir at room temperature to precipitate a solid. Filter, wash and dry. Recrystallized ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com