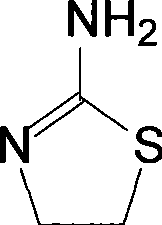
Method for synthesizing 2-amino thizaoline
A technology for the synthesis of aminothiazolines and methods, which is applied in the field of synthesis of 2-aminothiazolines, can solve the problems of many operation steps and high energy consumption, and achieve the effects of advanced process routes, low production costs, and shortened reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
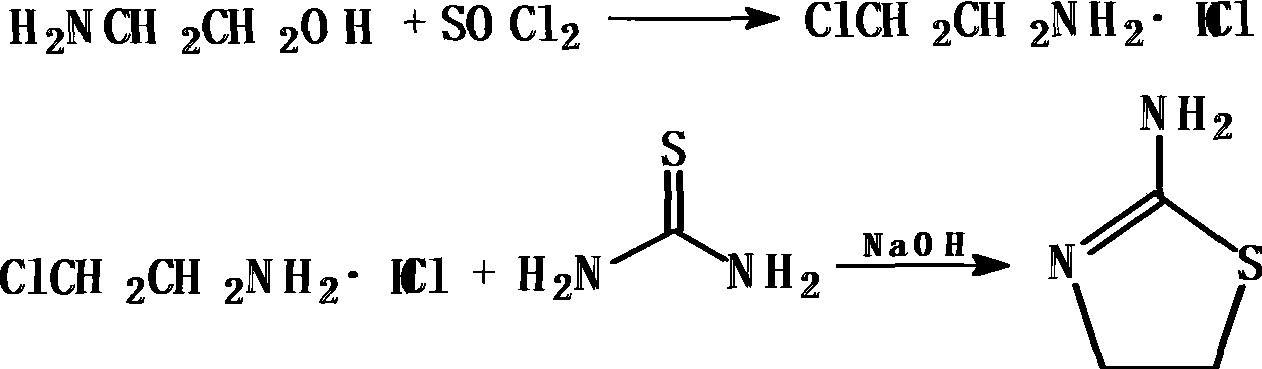
[0017] Preparation of 2-chloroethylamine hydrochloride
[0018] Add 250mL of chloroform and 46.5g (0.76mol) of ethanolamine into a dry reaction flask equipped with a reflux device, stirring and thermometer, start stirring, mix well, cool down to 10°C, and slowly add 121g (1.02mol) of chlorinated chlorinated The sulfone was added dropwise within 3 hours, and the temperature was raised to reflux temperature. After 15 hours of reaction, chloroform and excess thionyl chloride were distilled off, cooled slowly to precipitate crystals, continued to cool down to 5°C, filtered, and vacuum-dried to obtain 85.6g 2-Chloroethylamine hydrochloride white flaky crystals.
[0019] mp: 146.3~147.1℃ (literature value is 147.5~148℃), yield 97%, purity 99.2% (GC method), IR (KBr): υ -NH 3500cm -1 , 3116cm -1 , 1582cm -1 ; C-N 1110cm -1 .
[0020] Synthesis of 2-aminothiazoline
[0021] Dissolve 85.6g (0.74mol) of 2-chloroethylamine hydrochloride in 200mL of water, add 160g (2.10mol) of th...
Embodiment 2
[0024] Add 300mL of toluene and 46.5g (0.76mol) of ethanolamine to a dry reaction flask equipped with a reflux device, stirring and thermometer, start the stirring, make it evenly mixed, cool down to 10°C, and slowly add 119g (1mol) of thionyl chloride dropwise , the dropwise addition was completed within 3 hours, and the reflux reaction was maintained for 10 hours. The toluene and unreacted thionyl chloride were evaporated under normal pressure, and then the solvent was evaporated to dryness under reduced pressure at 60°C.
[0025] Add 200 mL of water and 165 g (2.17 mol) of thiourea to the residue in the reaction flask, heat to reflux for 20 hours, cool down to 70°C, add 40% sodium hydroxide solution to adjust the pH to 9, and extract with 200 mL of dichloromethane 2 times, and then extracted 1 time with 100mL dichloromethane, combined the extracts, dried over anhydrous sodium sulfate, concentrated, filtered, and vacuum dried to obtain 56.6g of white crystals of 2-aminothiazo...
Embodiment 3
[0027] Add 220mL of dichloromethane and 46.5g (0.76mol) of ethanolamine into a dry reaction flask equipped with a reflux device, stirring and thermometer, start stirring, mix well, cool down to 10°C, and slowly add 135g (1.13mol) of chlorine The thionyl chloride was added dropwise within 3 hours, and the reflux reaction was maintained for 12 hours, dichloromethane and thionyl chloride were evaporated under normal pressure, and distilled to dryness under reduced pressure.
[0028] Add 200 mL of water and 172 g (2.26 mol) of thiourea to the residue in the reaction flask, heat to reflux for 22 hours, cool down to 70°C, add 40% sodium hydroxide solution to adjust the pH to 9, and extract with 200 mL of dichloromethane 2 times, and then extracted 1 time with 100mL dichloromethane, combined extracts, dried over anhydrous sodium sulfate, concentrated, filtered, and vacuum-dried to obtain 57.2g of white crystals of 2-aminothiazoline, with a yield of 73.6% (relative to ethanolamine) , ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com