High-thermal stability two-component organic gel and preparation method thereof
A high thermal stability, organogel technology, applied in the field of supramolecular chemistry and functional materials, can solve problems such as thermal stability performance of gelling factors, complex preparation of gelling factors, complex chemical structure, etc., and achieve high gelation. -The effect of solution phase transition temperature, simple preparation method and fast preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 856mg of 2-ethylhexylamine, 500mg of trimesoyl chloride, and 670mg of triethylamine, dissolve them in 150mL of dry chloroform, and stir at 35°C for 12h. After the reaction solution was lowered to room temperature, extracted three times with deionized water, collected the organic phase, dried with anhydrous sodium sulfate, filtered and spin-dried the solvent under reduced pressure to obtain the first gel factor N 1 , N 3 , N 5 - Tris(2-ethylhexyl)benzene-1,3,5-tricarboxamide 813mg white powder solid, yield 79%.
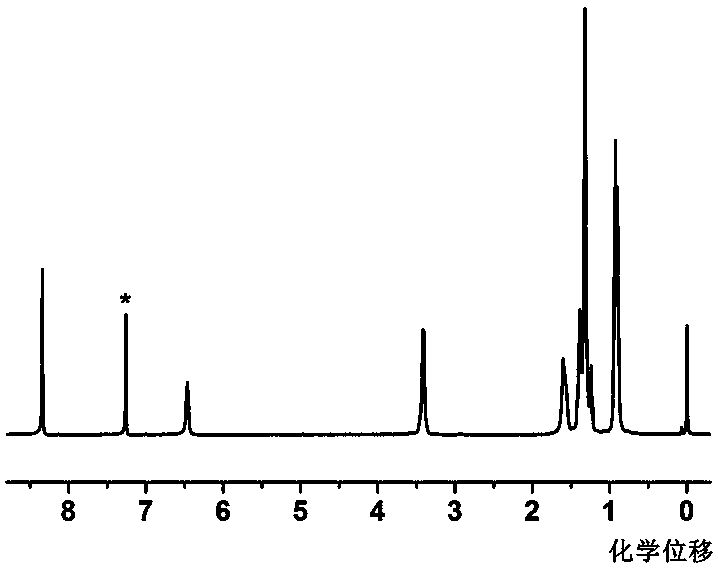
[0035] figure 1 for N 1 , N 3 , N 5-Proton NMR spectrum of tris(2-ethylhexyl)benzene-1,3,5-tricarboxamide. The * in the spectrogram indicates the solvent peak, and all the characteristic peaks are given clear assignments, and there are no miscellaneous peaks in the spectrogram, which proves that the prepared first gel factor has a high purity.
Embodiment 2
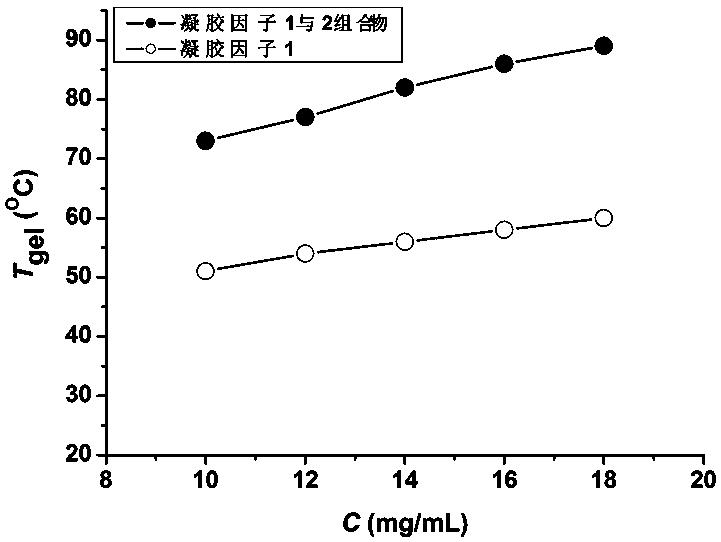
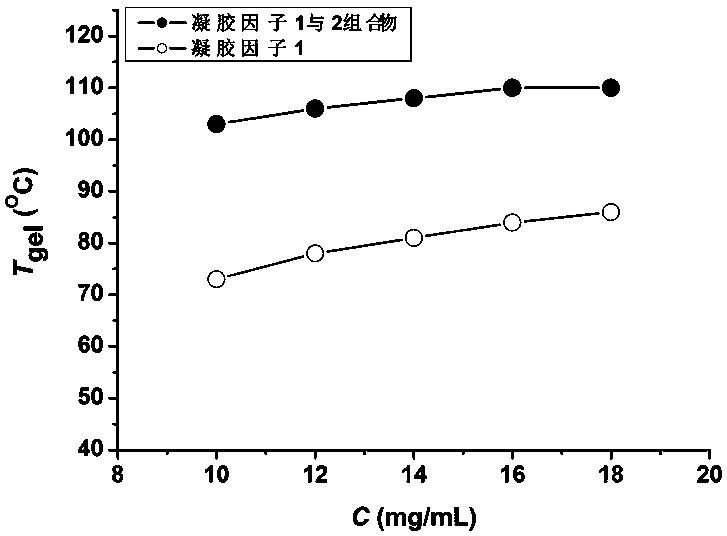
[0037] Weigh 9.5mg of the first gelling factor and 0.5mg of the second gelling factor into a screw-top vial with a diameter of 12mm, add 1mL of toluene, heat to 85°C and ultrasonically until the gelling factor is completely dissolved in toluene, then cool naturally To room temperature, let it stand for 9h to form a stable organogel.
[0038] The vial was placed upside down in a vacuum oven and heated at a rate of 12°C / h, and the gel-solution phase transition temperature of the organogel was measured to be 73°C.
[0039] Using the first gelling factor alone, an organogel is formed in toluene under the same conditions, and its gel-solution phase transition temperature is 51°C.
Embodiment 3
[0041] Weigh 11.4mg of the first gelling factor and 0.6mg of the second gelling factor into a screw-top vial with a diameter of 12mm, add 1mL of toluene, heat at 88°C and use strong ultrasonic waves until the gelling factor is completely dissolved in toluene, then cool naturally to After standing at room temperature for 8 hours, a stable organic gel was formed.
[0042] The vial was placed upside down in a vacuum oven and heated at a rate of 12°C / h, and the gel-solution phase transition temperature of the organogel was measured to be 77°C.
[0043] Using the first gelling factor alone, an organogel was formed in toluene under the same conditions, and its gel-solution phase transition temperature was measured to be 54°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com