Novel method for preparing ramelteon key intermediate
A technology for ramelteon and intermediates, applied in the field of chemical and pharmaceutical industries, can solve the problems of low yield and achieve the effects of simple post-treatment, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
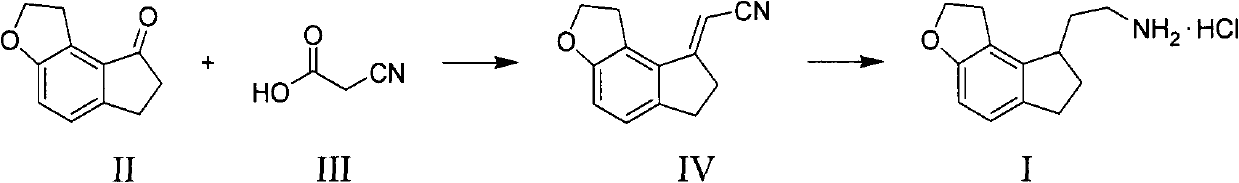
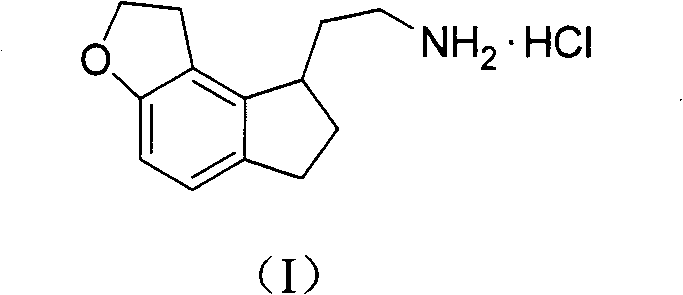
[0026] Example: 2-(1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-yl)ethylamine hydrochloride (I)
[0027] step 1):
[0028] 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (II) (34.8g, 0.200mol), cyanoacetic acid (III) (25.5g, 0.300mol ) and piperidine (0.084g, 0.986mmol) were added to toluene (160ml), stirred, and heated to reflux. After the reaction of compound (II) is complete, filter, concentrate the solvent under reduced pressure, add water (140ml), extract with ethyl acetate (50ml×3), wash with saturated aqueous sodium chloride until neutral, concentrate the solvent under reduced pressure to obtain a solid compound (IV) (36.4 g, 92.4%).
[0029] Melting point: 144-147°C
[0030] Step (2):
[0031] Add compound (IV) (34.5g, 0.175mol), Raney nickel (6.9g) and ammonia-saturated ethanol solution (800ml) into a 2L autoclave, and feed hydrogen to 40kg / m 2 , heated to 40 ° C, stirred for 5h. Filter, add 10% palladium carbon (3.6g) to the filtrate, pass hydrogen to 40kg / m 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com