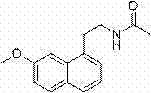
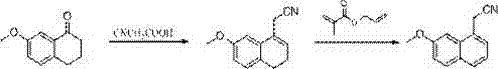
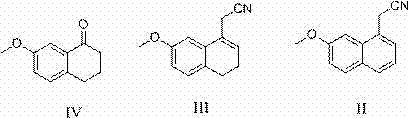
Synthetic method of 2-(7-methoxyl-1-naphthyl) ethylamine hydrochloride
A technology of ethylamine hydrochloride and methoxy group is applied in the field of synthesis of 2-(7-methoxy-1-naphthyl)ethylamine hydrochloride, which can solve problems such as being difficult to realize industrialized production, and achieve the steps of Concise, high reaction yield, beneficial to the effect of industrialization promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of (7-methoxy-3,4-dihydro-1-naphthyl)acetonitrile
[0034] At room temperature, successively put 176 g of 7-methoxytetralin-1-one, 128 g of cyanoacetic acid, 1600 ml of toluene, 27 g of benzylamine, and 33 g of heptanoic acid into a 2L reaction flask, start stirring, and heat for 114 Reflux at ℃ to separate water for about 16 hours. After all the substrates have reacted, stop heating. After cooling down to room temperature naturally, cool down in an ice-water bath to below 10℃ to precipitate a pale yellow solid. Filter and wash the filter cake with toluene. Wash the filtrate with 500ml, 2mol / L sodium hydroxide solution, separate the toluene phase, wash the toluene phase with 500ml purified water again, separate the liquids, and finally wash the toluene phase with 500ml saturated saline, separate the liquids, and put the toluene phase at 70- Concentrate to dryness under reduced pressure at 75°C, and the remaining brown oil is directly used in th...
Embodiment 2
[0035] Embodiment 2: the synthesis of (7-methoxy-1-naphthyl) acetonitrile
[0036] At room temperature, dissolve 100 g of (7-methoxy-3,4-dihydro-1-naphthyl)acetonitrile in 500 ml of dichloromethane, and the solution turns reddish brown. Add 125 g of DDQ and 1000ml of dichloromethane into a 2L reaction flask in turn, start stirring, heating, and add the dichloromethane solution of intermediate I dropwise at a controlled temperature of 25-28°C, and keep warm at 30±2°C for 1 hour after dropping. After heat preservation, filter, and wash the filtrate 3 times with 500ml saturated sodium bicarbonate solution, separate the dichloromethane layer, wash with 500ml water again, and separate the layers; finally wash the dichloromethane phase with 500ml saturated saline, and separate the layers. The dichloromethane phase was concentrated to dryness under reduced pressure at 30-35°C, and the remaining yellow oil was dissolved by adding 260ml of ethanol, cooled in an ice-water bath to cool...
Embodiment 3
[0037] Example 3: Synthesis of 2-(7-methoxy-1-naphthyl)ethylamine hydrochloride
[0038] At room temperature, dissolve 80 g of (7-methoxy-1-naphthyl)acetonitrile in 800 ml of anhydrous tetrahydrofuran, add 90 g of calcium chloride, control the temperature at 0-5°C, and add 31 g of sodium borohydride in batches , After the addition, rise to room temperature and react for 5h. After the reaction was completed and filtered, the filtrate was concentrated to dryness under reduced pressure at 50°C, and the remaining yellow oil was dissolved by adding 320ml of ethyl acetate, filtered hot, and about 30ml of hydrochloric acid was added to adjust the pH to 2-3, cooling and crystallization, stirring at 10°C for 1 -2h, filter, and dry the filter cake at 50°C to obtain 2-(7-methoxy-1-naphthyl)ethylamine hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com