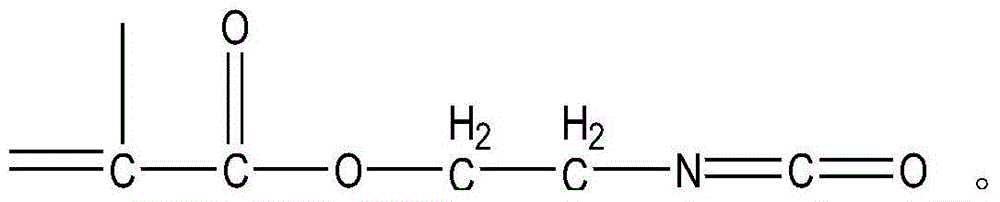
Preparation method for isocyanate ethyl methacrylate
A technology of methacrylic acid isocyanic acid and methacrylic acid is applied in the preparation of isocyanic acid derivatives, the preparation of carboxylic acid amides, the preparation of organic compounds, etc., and can solve problems such as unfavorable industrial production, low yield, and complicated steps. problem, to achieve the effect of simple and practical separation process, reduced safety hazards, and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) In a three-neck flask equipped with stirring, thermometer, condenser and tail gas absorption device, add 61g (1mol) of ethanolamine, 86g (1mol) of methacrylic acid, and 92g (1mol) of toluene, stir and heat up to 50°C, and slowly add 119g (1mol) of thionyl chloride was reacted, and the tail gas produced during the reaction was absorbed by the alkali solution. After the dropwise addition, the reaction was continued for 2 hours; distilled water was added to the generated product to stir and layered, and the obtained water layer was concentrated and dried Obtain 109g of methacryloyl (2-hydroxy)ethylamine hydrochloride; (2) Add 65.2g of solid phosgene, 60.6g of toluene and 0.3g of catalyst triethylamine into the flask, mix and slowly add methacryloyl (2-Hydroxy) ethylamine hydrochloride 109g, the tail gas that produces in the reaction process is absorbed by lye, dropwise is completed, and temperature rises to 80 ℃, continues reaction 3 hours; Carry out vacuum distillation...
Embodiment 2
[0023] (1) In a three-necked flask with stirring, thermometer, condenser and tail gas absorption device, add 61g (1mol) of ethanolamine, 86g (1mol) of methacrylic acid, and 450g (5mol) of dimethyl carbonate, and stir to heat up to 60°C. Slowly add 238g (2mol) of thionyl chloride dropwise to react, the tail gas generated during the reaction is absorbed by the lye, after the dropwise addition, continue to react for 4 hours; add distilled water to the product to stir and separate, and the obtained water layer is concentrated Dry to obtain methacryloyl (2-hydroxyl) ethylamine hydrochloride 112.9g; (2) add solid phosgene 202.6g, carbon tetrachloride 315g and catalyst dimethylformamide (DMF) 1g in the flask, Mix and stir and slowly add 112.9 g of methacryloyl (2-hydroxy) ethylamine hydrochloride dropwise. The tail gas generated during the reaction is absorbed by the alkali solution. After the dropwise addition, the temperature rises to 90°C and the reaction is continued for 2 hours; ...
Embodiment 3
[0025] (1) In a three-necked flask with stirring, thermometer, condenser and tail gas absorption device, add 61g (1mol) of ethanolamine, 86g (1mol) of methacrylic acid, and 990g (10mol) of dichloroethane, and stir to heat up to 80°C. Slowly add 357g (3mol) of thionyl chloride dropwise to react, the tail gas generated during the reaction is absorbed by the lye, after the dropwise addition, continue to react for 3 hours; add distilled water to the product to stir and separate, and the obtained water layer is concentrated Dry to obtain 106.5 g of methacryloyl (2-hydroxy) ethylamine hydrochloride; (2) Add 63.7 g of solid phosgene, 191.1 g of dichloroethane and 1.5 g of catalyst pyridine into the flask, mix and slowly add Methacryloyl (2-hydroxy) ethylamine hydrochloride 106.5g, the tail gas produced in the reaction process was absorbed by the lye, the dropwise addition was completed, the temperature was raised to 100°C, and the reaction was continued for 3 hours; then vacuum distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com