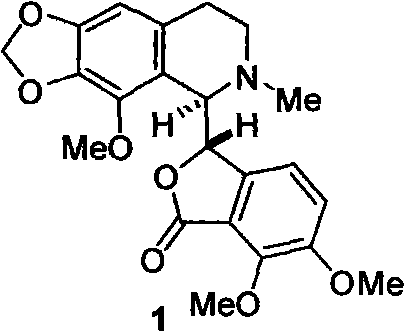
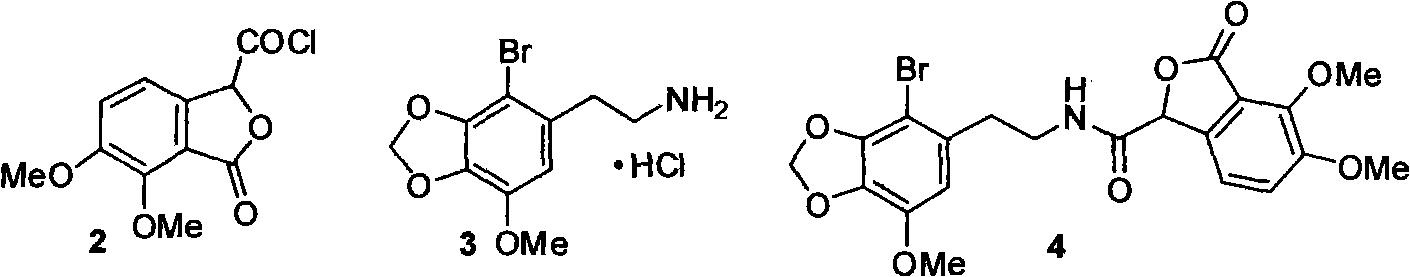
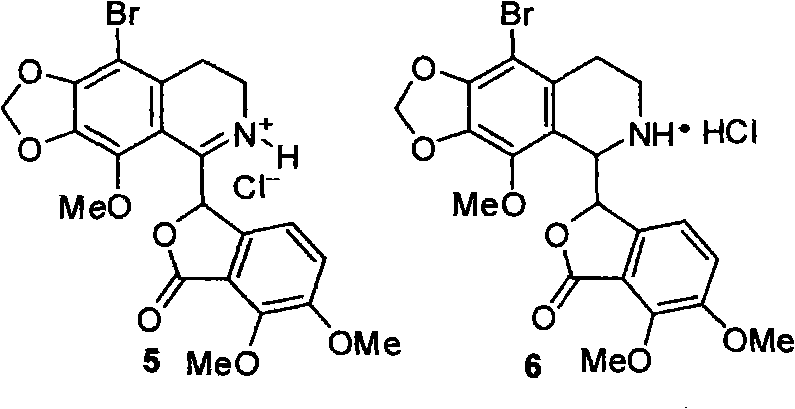
Method for selective synthesis of alpha-narcotine with participation of blockage group
A noscapine and selective technology, applied in organic chemistry and other fields, can solve problems such as unsatisfactory yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: N-(2-(2-bromo-3,4-methylenedioxy-5-methoxyphenyl)ethyl)-6,7-dimethoxy-1-oxo-isophenyl Preparation of furan-3-carboxamide
[0045] Under ice bath and stirring, 2-(2-bromo-3,4-methylenedioxy-5-methoxyphenyl)ethylamine hydrochloride 3.1g (10.0mmol), ether 20.0mL and 5.0% To a mixed solution of 42.0 mL of sodium carbonate solution, a mixed solution of 2.4 g (10.0 mmol) of acid chloride of 6,7-dimethoxyphthalide-3-carboxylic acid and 20.0 mL of ether was added dropwise, and a white solid gradually precipitated. After addition, continue to stir for 1.0h, and filter with suction to obtain some solids. The organic phase in the filtrate was separated, washed with saturated brine (40 mL), dried, filtered, and concentrated. The residue was combined with the white solid obtained by suction filtration, accumulatively 3.2 g, yield 65.5%.
[0046] MS(m / z): 493.0[M+H] + . 1 H NMR (CDCl 3 )δ2.86(t, 2H), 2.50(q, 2H), 3.84(s, 3H), 3.93(s, 3H), 4.10(s, 3H), 5.63(s, 1H), 6.0...
Embodiment 2
[0048] Example 2: 6,7-dimethoxy-3-(9-bromo-4-methoxy-5,6,7,8-tetrahydro-1',3'-dioxolane[4' , 5'-g]-5-isoquinolinyl)-1 (3H)-isobenzofuranone hydrochloride preparation
[0049] Under argon protection and stirring, N-(2-(2-bromo-3,4-methylenedioxy-5-methoxyphenyl)ethyl)-6,7-dimethoxy-1-oxo A mixture of 2.47 g (5 mmol) of oxo-isobenzofuran-3-carboxamide and 10.0 mL (10.0 mmol) of phosphorus oxychloride was heated at 100° C. for 1.5 h. After the excess phosphorus oxychloride was distilled off under reduced pressure, 20.0 mL of methanol was added to the residue to destroy the remaining phosphorus oxychloride, and the residue was concentrated to dryness under reduced pressure. The residue was dissolved by heating with 8.0 mL of methanol, and solid was precipitated by cooling. Suction filtration, drying to obtain light yellow solid.
[0050] Under stirring in an ice bath, the above light yellow solid was dissolved in 20 mL of methanol, and then 0.57 g (15 mmol) of sodium borohydrid...
Embodiment 3
[0052] Example 3: 6,7-dimethoxy-3-(9-bromo-4-methoxy-6-methyl-5,6,7,8-tetrahydro-1',3'-diox Preparation of pentacyclo[4',5'-g]-5-isoquinolinyl)-1(3H)-isobenzofuranone hydrochloride
[0053]Under stirring, 6,7-dimethoxy-3-(9-bromo-4-methoxy-5,6,7,8-tetrahydro-1',3'-dioxolane[4 ', 5'-g]-5-isoquinolinyl)-1(3H)-isobenzofuranone hydrochloride 0.51g (1.0mmol) was added to 37% formaldehyde aqueous solution 0.50mL (7.0mmol) and 88% In a mixed solution of 0.5 mL (12.0 mmol) of formic acid, the temperature was gradually raised to 100° C., and the reaction was carried out for 8 h. Cool to room temperature, add 2.0 mL of 6.0 mol / L hydrochloric acid, and continue stirring for 2 h. Concentrate to dryness under reduced pressure, add 10.0 mL of water to the residue to adjust pH=9, and extract with ethyl acetate (10.0 mL×2). The organic layers were combined, washed with saturated brine (10 mL×2), and dried over anhydrous sodium sulfate. After filtration and concentration, the obtained crud...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com