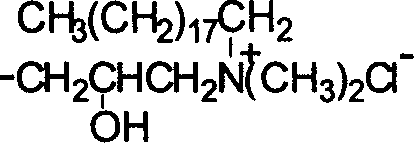Water and oil soluble O-chitosan derivatives and their preparation and use
A chitosan derivative, chitosan technology, applied in the direction of drug combination, organic active ingredients, medical preparations containing active ingredients, etc., to achieve the effect of improving absorption efficiency and obvious weight loss effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
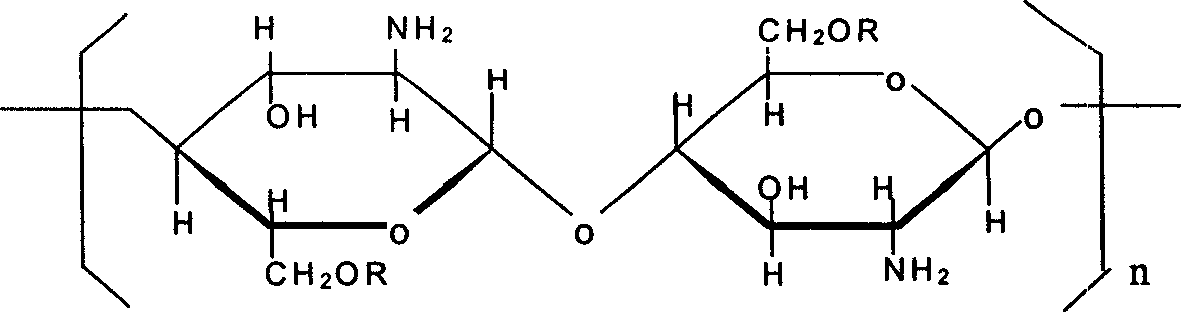
[0018] Embodiment one, the preparation of O-ethylamine hydroxyethyl chitosan
[0019] 1. Radiation degradation of chitosan: Chitosan with a molecular weight of 1.08 million and a degree of deacetylation of 85% is produced by Co 60 The radiation source is irradiated with a radiation dose of 60kGy to obtain chitosan oligomer A with a molecular weight of 100,000-200,000 and a deacetylation degree of 86%.
[0020] 2. Preparation of O-ethylamine hydroxyethyl chitosan:
[0021] 1. the preparation of N-phthaloyl chitosan: 0.83g phthalic acid solution is dissolved in the N, N-dimethylformamide that contains 6ml volume ratio of 5% water, adds 0.3g chitosan, in Stir and heat to 120°C under nitrogen. After reacting for 8 hours, the light brown mixture was cooled to room temperature and then poured into ice water. After filtration, the precipitate was washed with 150 ml of methanol for 1 hour and dried to give 0.444 g of substance B as a light brown powder.
[0022] ②Preparation of O-...
Embodiment 2
[0024] Embodiment two, O-2'-hydroxypropyl-N, the preparation of N-dimethyl octadecyl ammonium chloride chitosan
[0025] 1. The radiation degradation of chitosan: carry out by the first step in embodiment one
[0026] 2. Preparation of N, N dimethyl octadecyl tertiary amine chitosan:
[0027] 1. the preparation of N-phthaloyl chitosan: carry out by the first process in the second step in the embodiment one
[0028] ②Synthesis of N,N dimethyl octadecyl tertiary amine hydrochloride
[0029] Prepare 0.1mol / l dilute hydrochloric acid, add it dropwise to 5.95g N, N dimethyl octadecadecyl amine with a constant pressure funnel, control the pH value of the system to 4-11, stir and react in a water bath at room temperature for about 2 hours, and then freeze-dry The milky white cream hydrochloride C.
[0030] ③Reaction of hydrochloride and epichlorohydrin
[0031] Add 1ml of epichlorohydrin dropwise to 3.3g of C, and react in a water bath at room temperature for 6-24 hours to obtain...
Embodiment 3
[0034] Embodiment three, the therapeutic effect of water and oil two-soluble O-chitosan derivatives on obese rats
[0035] 1. Feed preparation and establishment of nutritional obesity model: Synthetic feed was selected as the basic animal feed, and whole egg powder, lard, cholesterol, milk powder and white sugar were added to the basic feed to adjust the energy, cholesterol and fat in the feed A high-fat feed was formulated and fed continuously for 6 weeks to form a nutritionally obese rat model.
[0036] 2. Animal grouping and research methods: After the model was successfully constructed, 70 obese animals were randomly divided into 7 groups according to body weight, 10 animals in each group. The diets fed to each group were as follows:
[0037] ① Obese mice control group (group C): fed with basic feed;
[0038] ②O-ethylamine hydroxyethyl chitosan (EAHECS) weight loss experimental group (TA1-3): TA1 group: basal feed + 2.5% EAHECS, TA2: basal feed + 5% EAHECS, TA3: basal fe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com