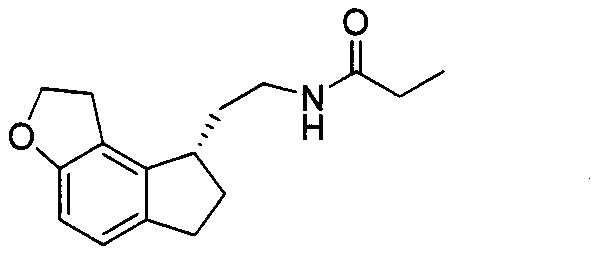
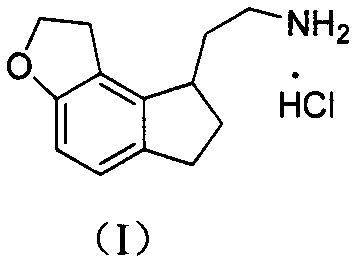
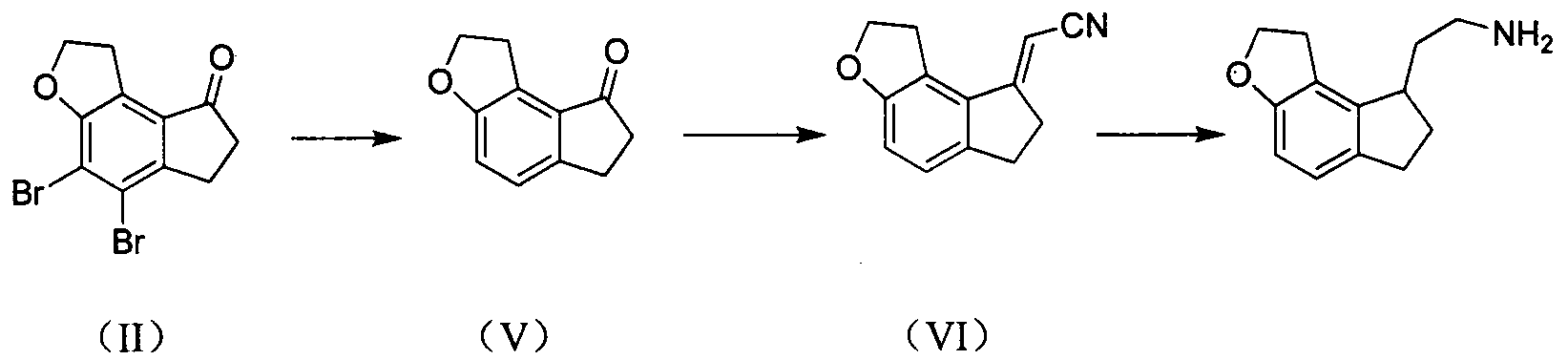
Preparation method of ramelteon intermediate
A technology of ramelteon and intermediates, which is applied in the field of preparation of ramelteon intermediates, can solve the problems of long reaction steps, complicated operation and high cost, and achieves the effects of shortening reaction steps, simple post-processing and avoiding generation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of (E)-2-(4,5-dibromo-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile (IV)
[0025] 4,5-dibromo-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (II) (33.20g, 0.100mol), cyanoacetic acid (III ) (21.30g, 0.250mol), benzylamine (2.68g, 0.025mol) and heptanoic acid (3.38g, 0.026mol) were added to toluene (300ml), heated to reflux. After the reaction of (II) was complete, cooling and crystallization gave 30.10 g of solid (IV) with a yield of 87.3%.
[0026] 1 H-NMR (CDCl 3 , 300MHz): δ6.68(1H, m), δ4.76(2H, t, J=17.5Hz), δ3.61(2H, d), δ3.53(2H, t, J=17.5Hz), δ3.40(2H,d).
Embodiment 2
[0028] Preparation of (E)-2-(4,5-dibromo-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile (IV)
[0029] 4,5-dibromo-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one (II) (16.60g, 0.050mol), cyanoacetic acid (III ) (12.75g, 0.150mol), phenethylamine (1.82g, 0.015mol) and added to toluene (135ml), heated to reflux. After the reaction of (II) was complete, cooling and crystallization gave 14.66 g of solid (IV), with a yield of 82.6%.
Embodiment 3
[0031] Preparation of 2-(1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-yl)ethylamine hydrochloride (I)
[0032] (IV) (28.4g, 0.080mol), sodium acetate (16.4g, 0.200mol), 10% palladium carbon (2.84g) and ethanol solution (580ml) are added in the autoclave, lead hydrogen to 4MPa, then be heated to 40 ° C, stirring for 8h. Filtrate, add Raney nickel (14.2g) and ammonia water (65ml) to the filtrate, pass hydrogen to 4MPa, heat to 40°C and stir for 4h. Filtrate, distill off the filtrate under reduced pressure to obtain a light yellow oil, add water (50ml) and ethyl acetate (150ml) and stir to dissolve, separate the ethyl acetate layer, dry over anhydrous sodium sulfate, filter, pass hydrogen chloride gas into the filtrate, Precipitation occurred. Filtration, the precipitate was washed with ethyl acetate and dried to obtain off-white solid 2-(1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-yl)ethylamine hydrochloride Salt (I) 14.5g, yield 75.6%.
[0033] 1 H-NMR (DMSO, 300MHz): δ1.55-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com