Preparation method of lapatinib intermediate
A technology for lapatinib and intermediates, applied in the field of preparation of lapatinib intermediate 2-thiamphenicol ethylamine hydrochloride, which can solve unfavorable large-scale production, hidden dangers, and Raney nickel catalytic hydrogenation reaction conditions Harsh and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
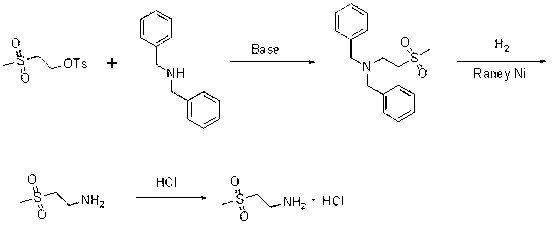
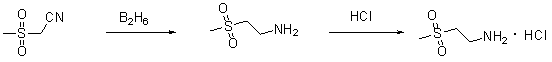
Method used
Image
Examples
example 1
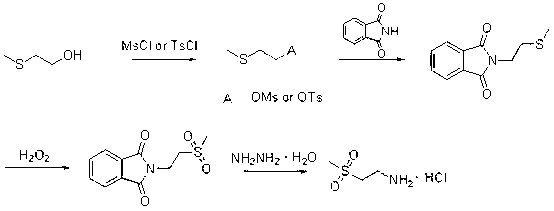
[0021] Put 2-methylthioethanol (92 g, 1.0 mol), triethylamine (141 g, 1.4 mol) and dichloromethane (1000 mL) in a 2000 mL three-necked reaction flask, cool down to 0~5 ℃, slowly drip A solution of methylsulfonyl chloride (148 g, 1.3 mol) in dichloromethane (300 mL) was added, and the temperature during the dropwise addition was controlled not to exceed 15 °C. After dripping, the temperature was raised to 30°C, and the reaction was carried out for 6 hours. Water (500 mL) was added, the layers were stirred, and the aqueous layer was added with dichloromethane (200 mL) for extraction. The organic layers were combined, and the solvent was evaporated under reduced pressure. The obtained oil was directly used in the next reaction.
[0022] The preparation method of N-methylthioethyl phthalimide
example 2
[0024] Put phthalimide (85 g, 0.58 mol), the oil obtained in Example 1, potassium carbonate (104 g, 0.75 mol) and N,N-dimethylformamide (800 mL) into 2000 mL three wells The reaction flask was heated to 100°C for 5 hours. Suction filtration while hot under reduced pressure, the filter cake was washed with N,N-dimethylformamide (100 mL), the filtrate was slowly added to ice water (2000 mL), and stirred for 30 minutes. Filter under reduced pressure and dry to obtain a white solid that is N-methylthioethylphthalimide (109 g, yield 85%).
[0025]
[0026] The preparation method of N-methylsulfonyl ethyl phthalimide
example 3
[0028] N-methylthioethylphthalimide (109 g, 0.49 mol) and glacial acetic acid (500 mL) were placed in a 2000 mL three-necked reaction flask, and the temperature was lowered to 5 °C. Slowly add 30% hydrogen peroxide (260 mL) dropwise, and control the reaction temperature not to be higher than 20 °C. After dripping, the reaction was refluxed for 7 hours. The temperature was lowered to 25°C, the reaction solution was slowly poured into ice water (2000 mL), stirred for 30 minutes, suction filtered, and dried to obtain white crystals (89 g, yield 72%).
[0029] The preparation method of 2-methylsulfonylethylamine hydrochloride
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com