Preparation method of lapatinib intermediate and analogues thereof
A compound and reaction time technology, applied in chemical recycling, organic chemistry, etc., can solve problems such as environmental pollution, waste of raw materials, and difficulty in recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The invention discloses a preparation method of a compound, comprising the following steps:
[0041] Step a) the compound shown in formula I is refluxed with 4-chloro-6-iodo-quinazoline in isopropanol,
[0042]
[0043] Formula I,
[0044] Wherein, R is a methyl group, a halogen substituent, an ester group or a cyano group;
[0045] Step b) diatomaceous earth, tin dichloride, trifluoroacetic acid, H 2 PdCl 4 Mix it with polyvinylpyrrolidone in water, heat it to 100-150°C, and get Pd catalyst after reaction;
[0046] Step c) the product obtained in step a), the Pd catalyst, 5-formylfuran boronic acid and K 2 CO 3 Mixing and reacting in the first organic solvent, the reaction temperature is 70-90°C;
[0047] Step d) mixing the reaction product obtained in step c), the organic base and 2-(thysulfonyl)ethylamine hydrochloride in a second organic solvent, and reacting to obtain a lapatinib intermediate or its analogue, the The lapatinib intermediate or its analogue...
Embodiment 1
[0065] Preparation of 5-iodo-2-aminobenzoic acid:
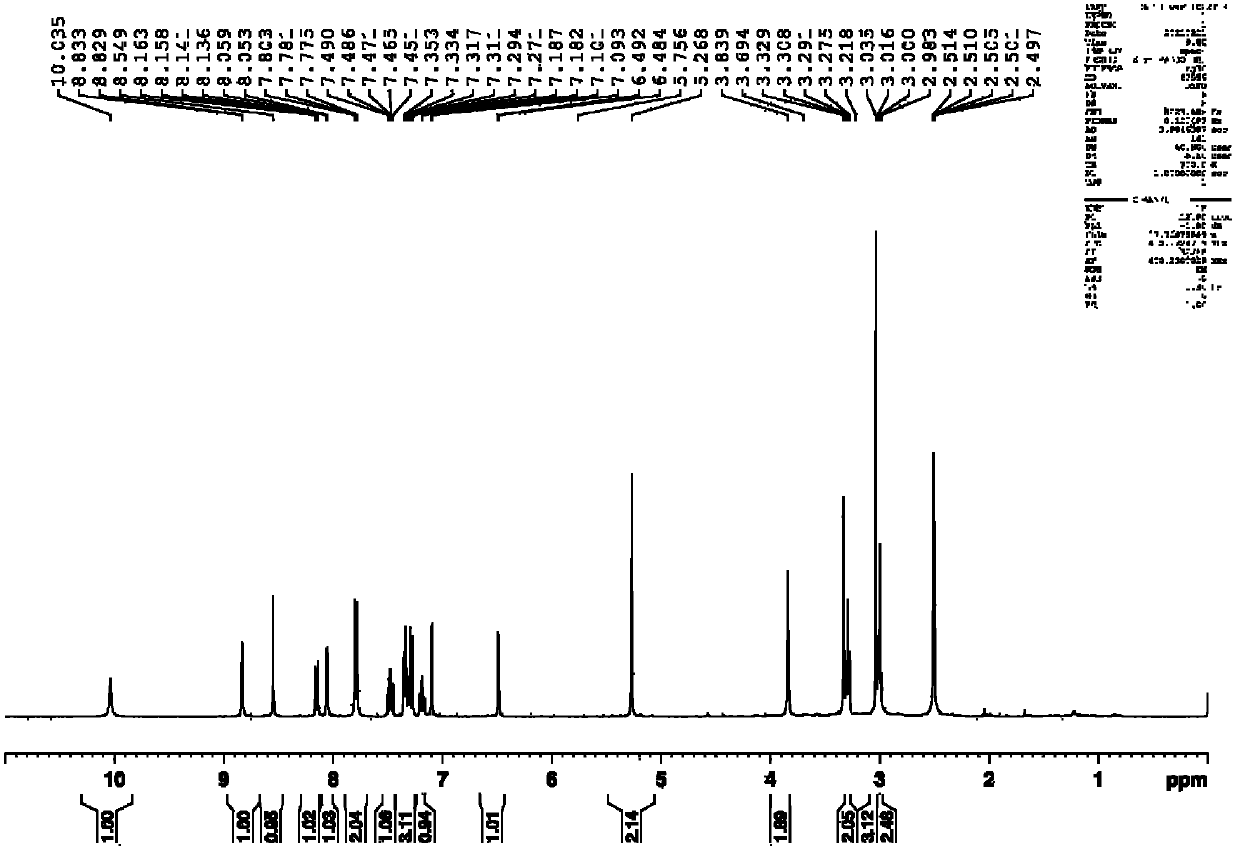
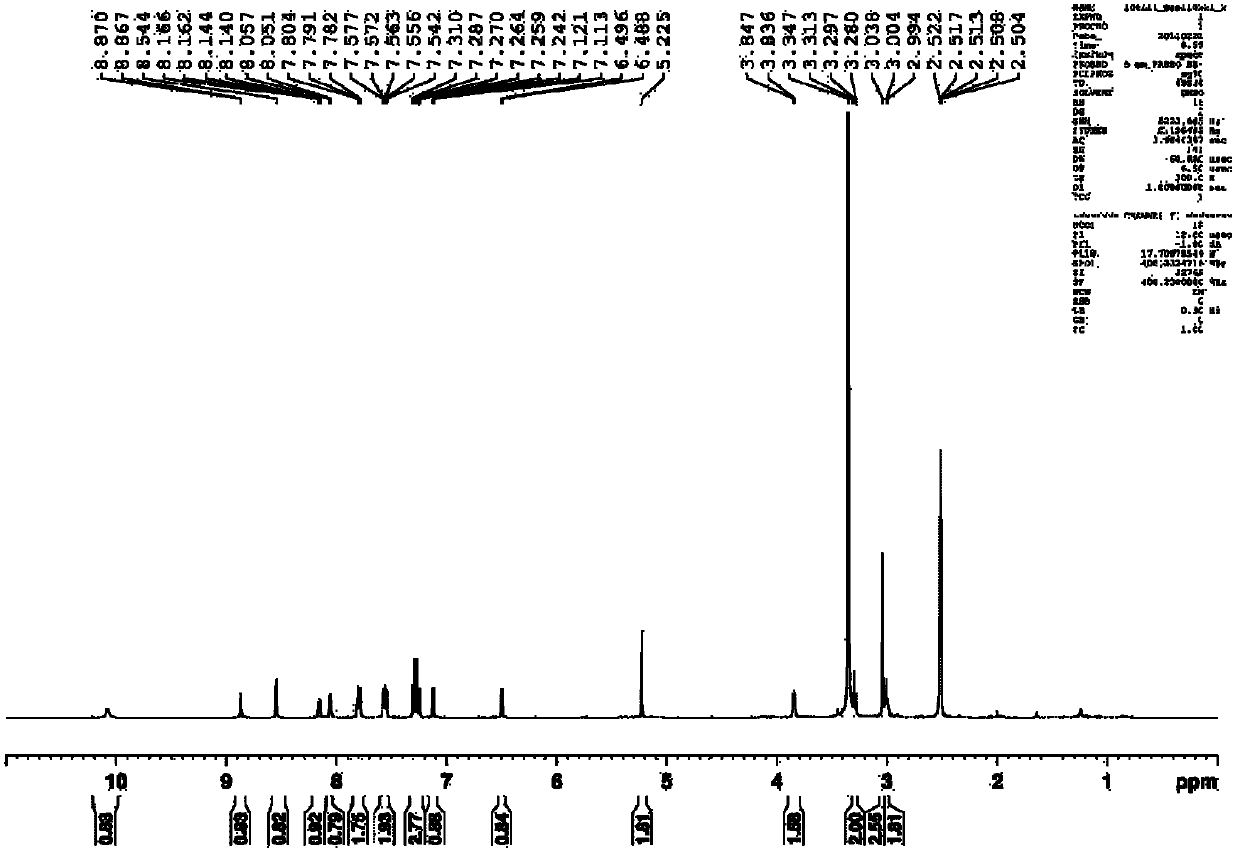
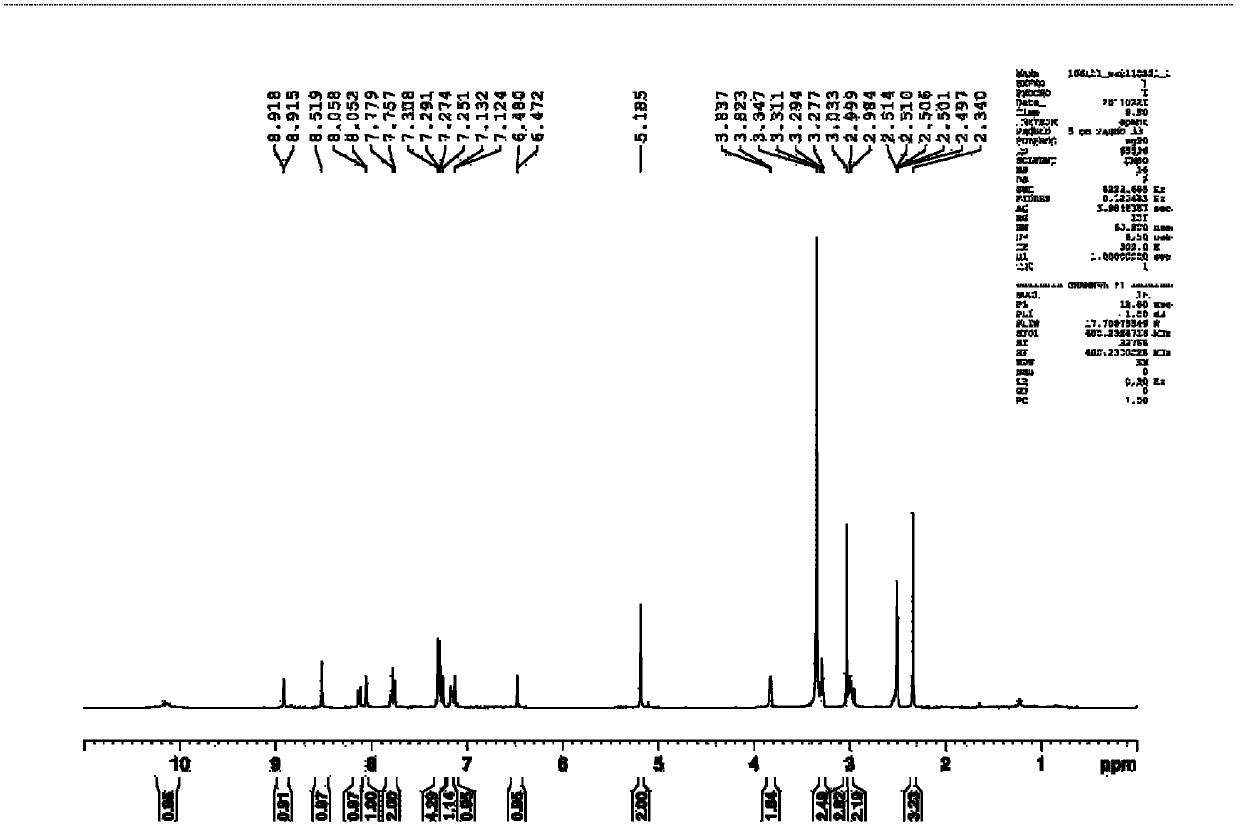
[0066] Anthranilic acid (10mmol, 1.37g), sodium periodate (10mmol, 2.14g) and sodium chloride (10mmol, 1.90g) were dissolved in 30mL of acetic acid aqueous solution (27mL of acetic acid, 3mL of water), and then slowly Add an aqueous solution (10mL) of potassium iodide (20mmol, 1.18g), and control the temperature within 50°C. After the addition, the reaction is stirred at room temperature for 8 hours, and then the reaction is stopped. The reaction system is poured into ice water, suction filtered, and the upper layer is filtered. The cake was eluted with a large amount of water and a small amount of ethanol, and the solid obtained by suction filtration was vacuum-dried at 60°C to finally obtain 2.52g of 5-iodo-2-aminobenzoic acid, yield: 95.7%. 1 HNMR (300MHz, DMSO-d 6 )δ9.50-8.13 (s, 2H), 7.91 (s, 1H), 7.45 (d, J=7.8Hz, 1H), 6.61 (d, J=7.8Hz, 1H). The reaction formula of the above-mentioned process is as follows:
[0067] ...
Embodiment 2
[0097] Preparation of 4-fluoro-benzyl alcohol:
[0098] Dissolve p-fluorobenzaldehyde (10mmol, 1.24g) in 25ml of methanol, then add sodium borohydride (10mmol, 0.37g) in batches, after the addition, the reaction system continues to react at room temperature for 6 hours, stop the reaction, and spin off the methanol , add water, then extract with ethyl acetate, dry over anhydrous sodium sulfate, spin off the solvent to obtain 1.19g of colorless liquid, yield: 94%, that is, 4-fluoro-benzyl alcohol, the reaction formula of the above process is as follows:
[0099]
[0100] Preparation of 2-chloro-1-(4-fluoro-benzyloxy)-4-nitrobenzene:
[0101] After dissolving 1,2-dichloro-4-nitrobenzene (5mmol, 0.960g) and p-fluorobenzyl alcohol (6mmol, 0.75g) prepared in Example 1 with DMF (5mL), then slowly Sodium hydride (7.5mmol, 180mg) was added slowly, after the addition, the reaction system was continued to react for 2 hours, the reaction was stopped, and then the system was poured int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com