Synthetic process of berberine
A synthesis process, a technology for berberine, applied in the direction of organic chemistry, etc., can solve the problems of difficult process, high cost, low yield, etc., and achieve the effects of avoiding cyanidation reaction, reducing toxicity, and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
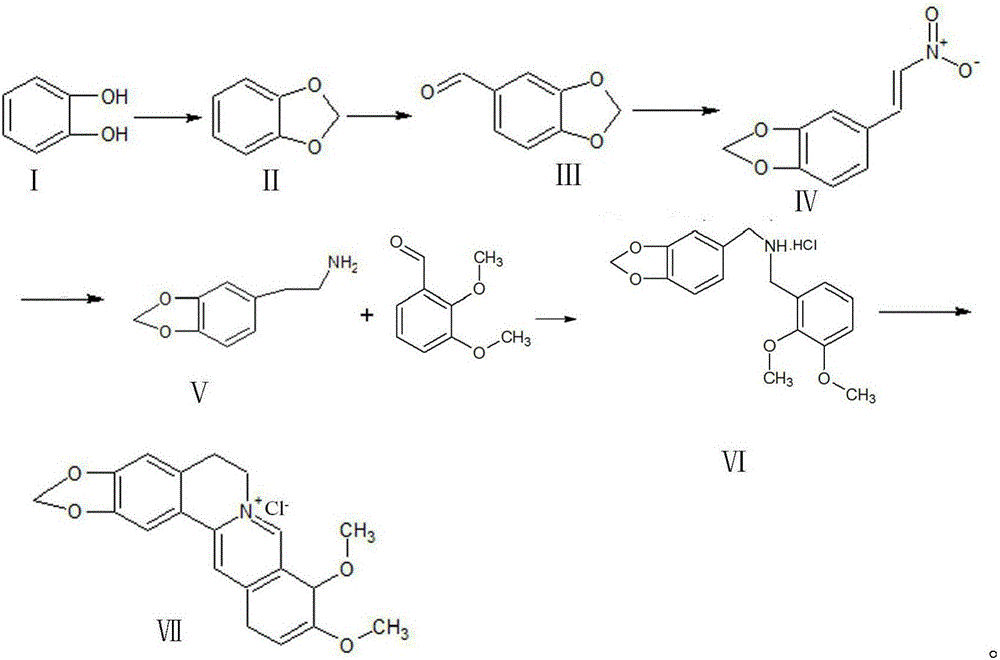
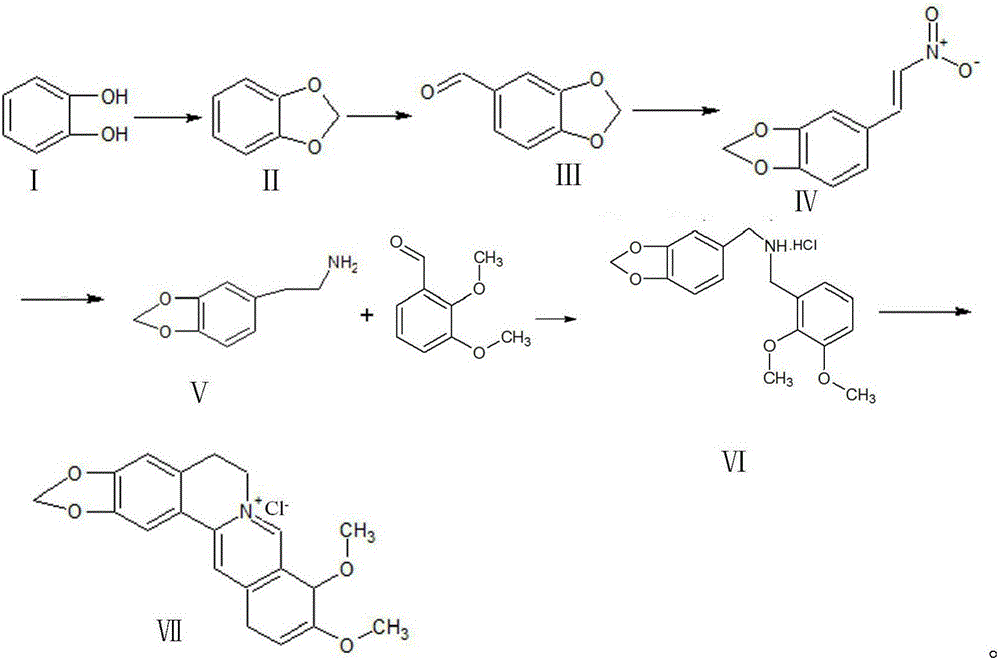
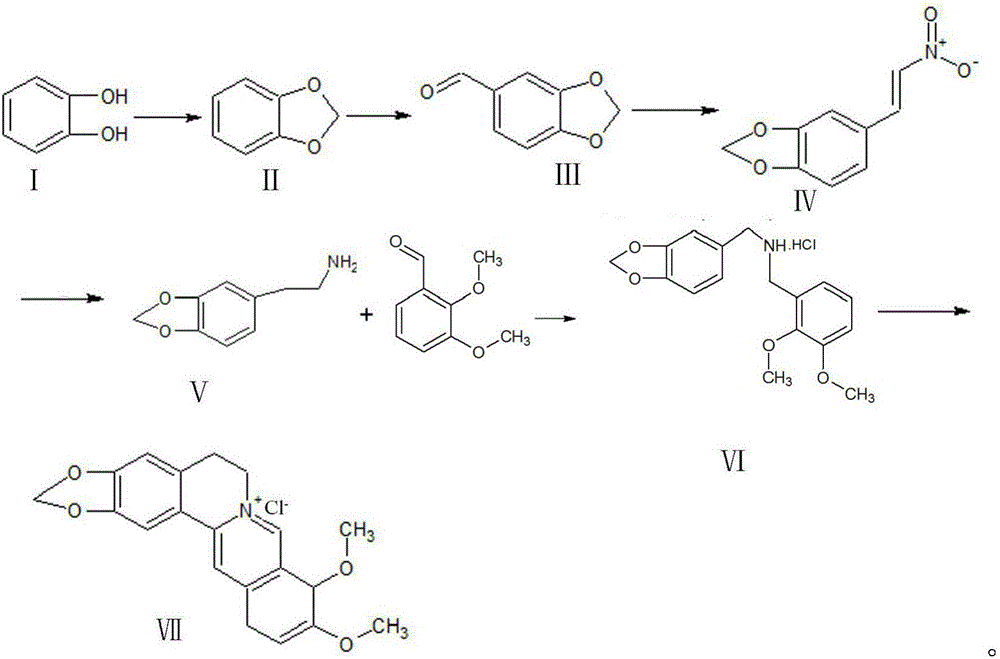
[0029] a. First, use catechol and dichloromethane as raw materials, under NaOH condition, dimethyl sulfoxide as solvent, and synthesize piperonyl ring at 95-120°C;
[0030] b. Peperonyl and dimethylformamide in POCl 3 Formylation occurs under conditions to synthesize piperonal;
[0031] c, piperonal and nitromethane undergo Henry reaction nitration under the conditions of sodium acetate, 95% ethanol and methylamine hydrochloride to generate β-nitro-3,4-dioxymethene styrene;
[0032] d, β-nitro-3,4-dioxymethene styrene is reduced to piperonyl ethylamine by zinc amalgam in 95% ethanol solution;
[0033] e, piperethylamine and 2,3-dimethoxybenzaldehyde in Raney nickel, H 2 Condensation hydrogenation under conditions to generate N-2,3-dimethoxybenzyl piperonyl ethylamine hydrochloride or piperonyl ethylamine and 2,3-dimethoxybenzaldehyde under the conditions of Pd / C ammonium formate and formic acid at 45- React at 60°C to generate N-2,3-dimethoxybenzylpiperethylamine hydrochlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com