One-step synthesis of ethyl isocyanate
A technology of ethyl isocyanate and trichloromethyl carbonate, applied in the field of chemistry, can solve the problems of pollution, high cost, dangerous degree of light-passing process and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] 1. Catalyst preparation: Mix commercially available alkylammonium salts and benzylammonium groups at a ratio of 1:4.2, reflux in ether solvent for 1 hour at the boiling point, cool down, centrifuge, dry, and pulverize for later use.
[0025] 2. Synthesis of the target product:
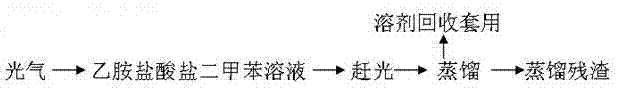
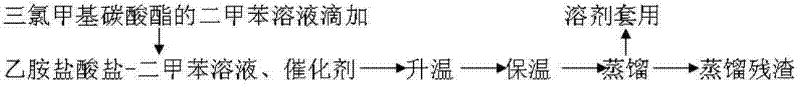
[0026] 180kg of ethylamine hydrochloride and mixed xylene were thrown into the reaction kettle and refluxed at 138°C to separate the water until the water content was ≤200ppm.
[0027] Cool down to below 40°C, add 8kg of catalyst and 260kg of trichloromethylcarbonate to rise through the program, keep warm to 135-137°C, add the xylene solution of trichloromethylcarbonate dropwise at 28kg / h at this temperature, the central control Analyze until the reaction is complete.
[0028] 3. Separation of the target product: Put the synthesis solution in a rectification kettle (number of plates: 40) and raise the temperature to reflux, completely remove the acid gas, and receive the target product at 60°C,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com