Preparation method of ramelteon
A technology of ethylamine and compounds, applied in the field of medicinal chemistry, can solve the problems of low total reaction yield and many reaction steps, and achieve the effect of simple operation, single type and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
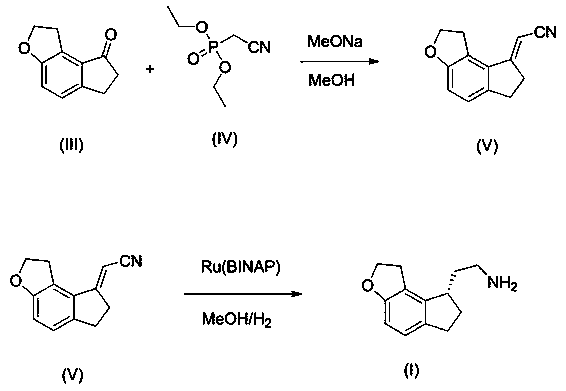
[0035] Weigh 100.00 g of 1,6,7,8-tetrahydro-8H-indeno[5,4-b]furan-8-one, 122.03 g of diethyl cyanomethylphosphonate, and 130.27 g of sodium methoxide. Keep the temperature at 35 °C, stir for 12 h, then add 2 L of water, stir for 4 h, then filter, wash the filter cake with 200 ml of water, and dry at 50 °C for 24 h. 98 g of white solid was obtained.
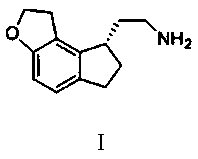
[0036] 90.00 g of the white solid obtained above was dispersed by adding 540 ml of methanol, and [Et 2 NH 2 ] + [Ru 2 Cl 5 (BINAP) 2 ] - 1.66 g. After the addition, pass hydrogen to 5 MPa and stir at 50 °C for 20 h. TLC detects that the reaction is complete. After the reaction is complete, the solvent is spin-dried, and then 450 ml of n-heptane is used for slurry, and then filtered. The solid cake is collected and dried at 50 °C for 1 h. In 270 ml of ethyl acetate and 270 ml of n-heptane, dissolved at 50 °C and then crystallized at 0 °C to obtain 90.15 g of yellow oil. The purity of the final product by HPLC was 98.5%.
Embodiment 2
[0038] Weigh 100.00 g of 1,6,7,8-tetrahydro-8H-indeno[5,4-b]furan-8-one, 122.03 g of diethyl cyanomethylphosphonate, and 130.27 g of sodium methoxide. Keep the temperature at 35 °C, stir for 12 h, then add 2 L of water, stir for 4 h, then filter, wash the filter cake with 200 ml of water, and dry at 50 °C for 24 h. 98 g of white solid was obtained.
[0039] 90.00 g of the white solid obtained above was dispersed by adding 540 ml of methanol, and [Ru (BINAP) (OCOCH 3 ) 2 ] 0.86 g, after the addition, pass hydrogen to 5 MPa and stir at 50 °C for 20 h, and the reaction is complete as detected by TLC. Spin to dry the solvent, then beat with 450 ml of n-heptane, then filter, collect the filter cake solids and dry at 50 °C for 1 h, then place in 270 ml of ethyl acetate and 270 ml of n-heptane, dissolve at 50 °C and crystallize at 0 °C 90.15 g of yellow oil were obtained. The purity of the final product by HPLC was 95.3%.
Embodiment 3
[0041] Weigh 100.00 g of 1,6,7,8-tetrahydro-8H-indeno[5,4-b]furan-8-one, 122.03 g of diethyl cyanomethylphosphonate, and 130.27 g of sodium methoxide. Keep the temperature at 35 °C, stir for 12 h, then add 2 L of water, stir for 4 h, then filter, wash the filter cake with 200 ml of water, and dry at 50 °C for 24 h. 98 g of white solid was obtained.
[0042] 90.00 g of the white solid obtained above was dispersed by adding 540 ml of methanol, and [RuCl(BINAP)( p -cymene)]Cl 1.26 g, after the addition was completed, pass hydrogen to 5 MPa and stir at 50 °C for 20 h, and TLC detected that the reaction was complete. Spin to dry the solvent, then beat with 450 ml of n-heptane, then filter, collect the filter cake solids and dry at 50 °C for 1 h, then place in 270 ml of ethyl acetate and 270 ml of n-heptane, dissolve at 50 °C and crystallize at 0 °C 90.15 g of yellow oil were obtained. The purity of the final product by HPLC was 96.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com