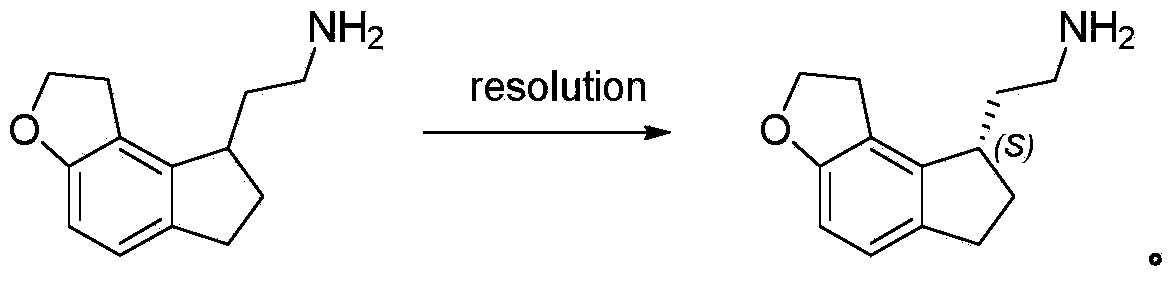
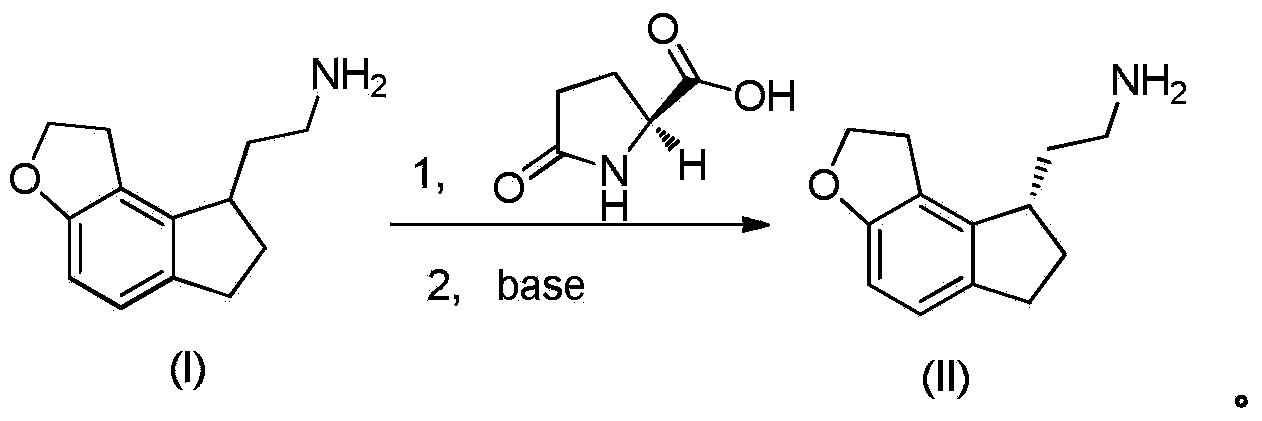
Resolution method of ramelteon intermediate
A technology for ramelteon and intermediates, applied in the field of resolution of ramelteon intermediates, can solve the problems of resolution yield and efficiency without detailed results, achieve good application prospects, easy operation, and controllable quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put S-ibuprofen (100g, 0.487mol, 1eq) and acetonitrile (1L) into the reaction flask, stir to dissolve it, and add 2,2-(1,6,7,8-tetrahydro-2H -Indeno[5,4-B]furan-8-yl)ethylamine (100g, 0.487mol, 1eq) in methanol (0.5L) solution, kept dropping within 30 minutes, after dropping, refluxed and stirred for two hours, Naturally cooled to room temperature, stirred at 0-5 degrees Celsius for 5 hours, filtered, and the filter cake was placed in a mixed solvent of acetonitrile and methanol (1L, v:v=6:4), heated at 80 degrees Celsius to dissolve it completely, and cooled naturally After crystallization, stir at 0-5 degrees Celsius for 5 hours, filter, add 1M aqueous sodium hydroxide solution to the filter cake to adjust the pH=10-12, stir for 15 minutes, filter, wash the filtrate to nearly neutral, and dry at 50 degrees Celsius to obtain White solid 35g, yield: 70%, ee: 97%.
Embodiment 2
[0022] Put S-ibuprofen (100g, 0.487mol, 1eq) and ethyl acetate (1L) into the reaction flask, stir to dissolve it, and add 2,2-(1,6,7,8-tetrahydro -2H-indeno[5,4-B]furan-8-yl)ethanamine (100g, 0.487mol, 1eq) in methanol (0.5L) solution, kept dripping within 30 minutes, and stirred under reflux for two Hours, naturally cooled to room temperature and then stirred at 0-5 degrees Celsius for 5 hours, filtered, the filter cake was placed in a mixed solvent of ethyl acetate and methanol (1L, v:v=6:4), heated to 80 degrees Celsius to dissolve it all After natural cooling and crystallization, stir at 0-5 degrees Celsius for 5 hours, filter, add 1M aqueous sodium hydroxide solution to the filter cake to adjust the pH=10-12, stir for 15 minutes, filter, wash the filtrate to nearly neutral, 50 degrees Celsius After drying, 30 g of white solid was obtained, yield: 60%, ee: 95%.
Embodiment 3
[0024] Put S-ibuprofen (100g, 0.487mol, 1eq) and tetrahydrofuran (1L) into the reaction flask, stir to dissolve it, and add 2,2-(1,6,7,8-tetrahydro-2H -Indeno[5,4-B]furan-8-yl)ethylamine (100g, 0.487mol, 1eq) in methanol (0.5L) solution, kept dropping within 30 minutes, after dropping, refluxed and stirred for two hours, Naturally cooled to room temperature, stirred at 0-5 degrees Celsius for 5 hours, filtered, and the filter cake was placed in a mixed solvent of tetrahydrofuran and methanol (1L, v:v=6:4), heated at 80 degrees Celsius to dissolve it completely, and cooled naturally. After crystallization, stir at 0-5 degrees Celsius for 5 hours, filter, add 1M aqueous sodium hydroxide solution to the filter cake to adjust the pH=10-12, stir for 15 minutes, filter, wash the filtrate to nearly neutral, and dry at 50 degrees Celsius to obtain White solid 28g, yield: 56%, ee: 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com