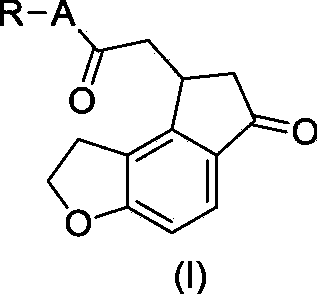
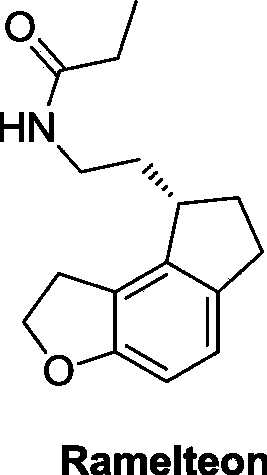
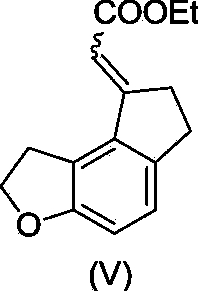
Critical intermediates used for preparing ramelteon, preparing method thereof and applications thereof
A technology of ramelteon and an intermediate, which is applied in the field of medicinal chemistry, can solve the problems of high cost, troublesome preparation of starting materials, etc., and achieves the effects of simple operation, novel synthesis route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The synthesis of embodiment 1.3-iodophenol or known as m-iodophenol (compound of formula 1)
[0072]
[0073] Dissolve m-aminophenol (100g, 961.4mmol) in 300mL10% HCl, cool in an ice bath to 0°C, slowly add (94.8g, 1.37mol) saturated sodium nitrite solution, stir for 10min, slowly add urea (44g, 733mmol) in batches, Stir for 10 minutes after addition, add dropwise KI (304g, 1.83mol) solution (500mL 1,2-dichloroethane and 500mL water mixed solvent), after dropwise reaction at room temperature for 1-2h, the reaction is complete, dichloromethane extraction, combined organic phase , washed with saturated sodium bicarbonate, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated, and beaten to obtain a brown solid (compound of formula 1, 181 g, yield 90%).
Embodiment 2
[0074] Example 2. Synthesis of 3-iodo-1-(2-bromoethoxy)benzene (compound of formula 2)
[0075]
[0076]Under the protection of argon, dissolve the raw material m-iodophenol (compound of formula 1, 66g, 300mmol) in acetone (350ml) as solvent, cool to 0°C in an ice bath, slowly add potassium carbonate (210g, 1500mmol), dropwise add 1,2- Dibromoethane (250mL, 3000mmol), react overnight at 55°C after dropping, complete reaction, cool, filter, concentrate at 40°C to remove acetone, concentrate at 45°C with oil pump to remove 1,2-dibromoethane, to obtain a brown liquid, stand still Overnight, it turned into a yellow solid, recrystallized from a mixed solvent of petroleum ether: ethyl acetate (10:1, volume ratio), precipitated impurities, filtered, concentrated the mother liquor, and separated by column chromatography to obtain a colorless liquid, which was cooled and stood to obtain White solid (namely the compound of formula 2, 83.3g, yield 85%).
[0077] Formula 2 compound: ...
Embodiment 3
[0079] Example 3. Synthesis of 2-iodo-4-(2-bromoethoxy)-acetophenone (compound of formula 3)
[0080]
[0081] Under the protection of argon, add the compound of formula 2 (100g, 305.85mmol) into a dry three-necked flask, dissolve the dry DCM (500ml), cool to 0°C in an ice bath, add acetyl chloride (29g, 367mmol), and slowly add AlCl in batches 3 (53g, 466mmol), add Bi to rise to room temperature and react for 1-3 hours, the reaction is complete, pour into ice water to quench the reaction, filter, extract the aqueous phase with DCM, combine the organic phases, concentrate, wash with saturated brine, anhydrous sulfuric acid Dry over sodium, filter and concentrate to give a pale yellow solid (crude).
[0082] Recrystallization: heat and dissolve an appropriate amount of ethyl acetate, add 5 times the volume of petroleum ether, let it stand overnight, precipitate crystals, filter, and dry to obtain white crystals (that is, the compound of formula 3, 53g, yield 47%).
[0083] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com