Preparation method of high-purity ramelteon
A ramelteon, high-purity technology, applied in the field of preparation of high-purity ramelteon, can solve the problems of affecting the total yield of the process and product quality, increasing production costs, difficulties, etc., to achieve the suppression of the formation of impurity VIII, Reduced purification pressure and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
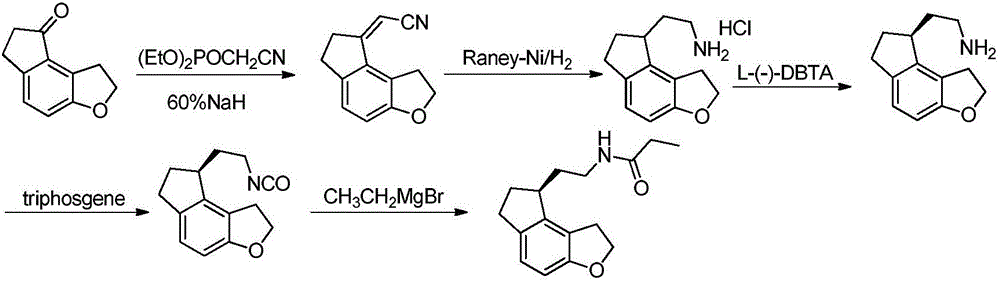
[0045]Add tetrahydrofuran (22L) and 60% NaH (2kg) into a 50L double-layer glass reactor and stir evenly below 25°C. Diethyl cyanomethylphosphonate (14kg) was added dropwise and stirred for 1 hour to obtain a clear ylide solution. Add tetrahydrofuran (22L) and 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone (formula (I) compound) in addition in 100L double-layer glass reaction kettle ( 4kg) and stir evenly. Add the freshly prepared ylide solution dropwise into the system, stir and react for 3 hours, then slowly add water (22 L) into the reaction system, and continue stirring for 30 minutes after the addition is complete. The reaction solution was poured into water (202 L) and stirred for 1 hour. Filtration gave 5.1 kg of 1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-ylideneacetonitrile (compound of formula (II)).
[0046] Add formula (II) compound (5kg), absolute ethanol (74L) and di-tert-butyl dicarbonate (11kg) in 300L glass-lined reaction tank and stir evenly, add sodium b...
Embodiment 2
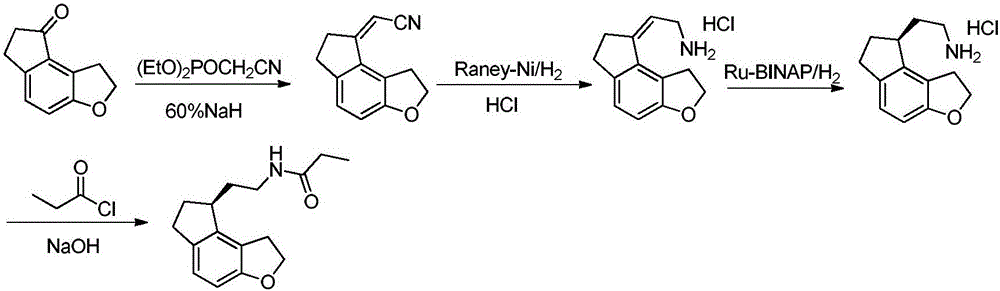
[0052] Add tetrahydrofuran (22L) and 60% NaH (2kg) into a 50L double-layer glass reactor and stir evenly below 25°C. Diethyl cyanomethylphosphonate (14kg) was added dropwise and stirred for 1 hour to obtain a clear ylide solution. Add tetrahydrofuran (22L) and 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone (formula (I) compound) in addition in 100L double-layer glass reaction kettle ( 4kg) and stir evenly. Add the freshly prepared ylide solution dropwise into the system, stir and react for 3 hours, then slowly add water (22 L) into the reaction system, and continue stirring for 30 minutes after the addition is complete. The reaction solution was poured into water (202 L) and stirred for 1 hour. Filtration gave 5.1 kg of 1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-ylideneacetonitrile (compound of formula (II)).
[0053] Add formula (II) compound (5kg), absolute ethanol (74L) and di-tert-butyl dicarbonate (11kg) in 300L glass-lined reaction tank and stir evenly, add sodium ...
Embodiment 3
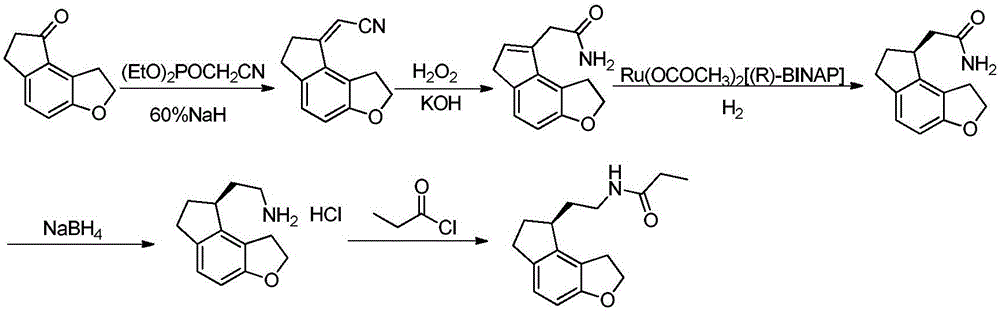
[0059] Add tetrahydrofuran (22L) and 60% NaH (2kg) into a 50L double-layer glass reactor and stir evenly below 25°C. Diethyl cyanomethylphosphonate (14kg) was added dropwise and stirred for 1 hour to obtain a clear ylide solution. Add tetrahydrofuran (22L) and 1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ketone (formula (I) compound) in addition in 100L double-layer glass reaction kettle ( 4kg) and stir evenly. Add the freshly prepared ylide solution dropwise into the system, stir and react for 3 hours, then slowly add water (22 L) into the reaction system, and continue stirring for 30 minutes after the addition is complete. The reaction solution was poured into water (202 L) and stirred for 1 hour. After filtration, 5.1 kg of 1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-ylideneacetonitrile (compound of formula (II)) was obtained.
[0060] Add formula (II) compound (5kg), absolute ethanol (74L) and di-tert-butyl dicarbonate (11kg) in 300L glass-lined reaction tank and stir even...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com