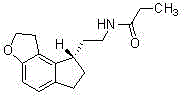
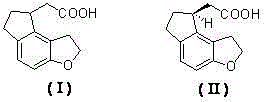Tricyclic acetate compound with optical activity, and preparation method and application thereof
An optically active compound technology, applied to optically active tricyclic acetate compounds, the application of ramelteon and its optical isomers, the field of salts formed by optically active 2-acetic acid and chiral amines , which can solve the problems of excessive heavy metals in the product, high preparation cost of ramelteon, and low total yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
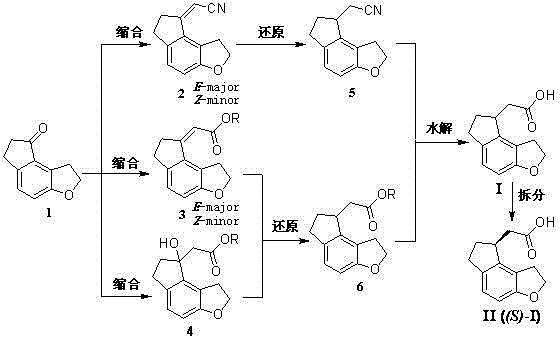
Image
Examples
Embodiment 1
[0064] (1,6,7,8-tetrahydro-2 H Preparation of -indeno-[5,4-b]furan-8-ylidene)acetonitrile (2)
[0065] Add 21.27 g (0.12 mol) of diethyl cyanomethylene phosphonate, 1,2,6,7-tetrahydro-8 H -Indeno-[5,4-b]furan-8-one 17.42 g (0.10 mol), toluene 120 ml and deionized water 60 ml, after stirring at room temperature, add tetrabutylammonium bromide 1.62 g (0.005 mol ) and 20.70 g (0.15 mol) of anhydrous potassium carbonate, heat up to 60-70°C, heat and stir for 8 h; after the reaction, cool to room temperature, separate the toluene layer, extract the water layer with 30 ml toluene, and combine the toluene solution , washed with 25 ml of saturated NaCl aqueous solution, the solvent was evaporated under reduced pressure, and the residue was recrystallized from methanol to obtain 17.20 g of light yellow crystalline solid, mp: 146~148°C, yield 87.2%.
Embodiment 2
[0067] (1,6,7,8-tetrahydro-2 H Preparation of -indeno-[5,4-b]furan-8-ylidene)acetonitrile (2)
[0068] Add 40.54 g (0.12 mol) of cyanomethylenetriphenylphosphonium chloride, 1,2,6,7-tetrahydro-8 H -Indeno-[5,4-b]furan-8-one 17.42 g (0.10 mol) and toluene 200 ml, after stirring at room temperature, cool to 0-5°C in an ice bath, add 15% sodium methoxide dropwise Methanol solution (0.13 mol), after dropping, remove the ice bath, stir at room temperature for 2.5 h, add 100 ml of deionized water into the reaction bottle, stir for 30 min, pour the reaction solution into a separatory funnel, separate the organic layer, And washed with 40 ml of saturated NaCl aqueous solution, the solvent was evaporated under reduced pressure, and the residue was recrystallized from methanol to obtain 16.51 g of light yellow crystalline solid, mp: 145~148 °C, yield 83.7%.
Embodiment 3
[0070] (1,6,7,8-tetrahydro-2 H Preparation of -indeno-[5,4-b]furan-8-ylidene)methyl acetate (3a)
[0071] The operation process is the same as in Example 1, except that diethyl cyanomethylene phosphonate is replaced with 2-(diethoxyphosphonic acid) methyl acetate to obtain (1,6,7,8-tetrahydro-2 H -indeno-[5,4-b]furan-8-ylidene)methyl acetate as light yellow oily liquid, yield 92.1%. It was directly used in the next reaction without further purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com