Ramelteon quick-release and slow-release double-release preparation and preparation method thereof
A ramelteon, dual-release technology, applied in the directions of pill delivery, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problem of premature awakening of patients, short duration of drug efficacy, and initial release of sustained-release preparations. Problems such as low stage rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] The present invention also provides a method for preparing the above-mentioned rapid-release sustained-release double-release preparation of ramelteon, comprising the following steps:
[0086] Take ramelteon, the first filler and the disintegrant and mix for the first time, then add the first lubricant and mix to prepare the immediate release part;
[0087] Take ramelteon, the second filler and the sustained-release material and mix them for the second time, then add the second lubricant and mix to prepare the sustained-release part.
[0088] In a specific example, the immediate-release part and the sustained-release part are used for double-layer compression.
[0089] In a specific example, after the first mixing, the following step is further included: performing wet granulation with ethanol or water.
[0090] In a specific example, after the second mixing, the following step is further included: performing wet granulation with ethanol or water.
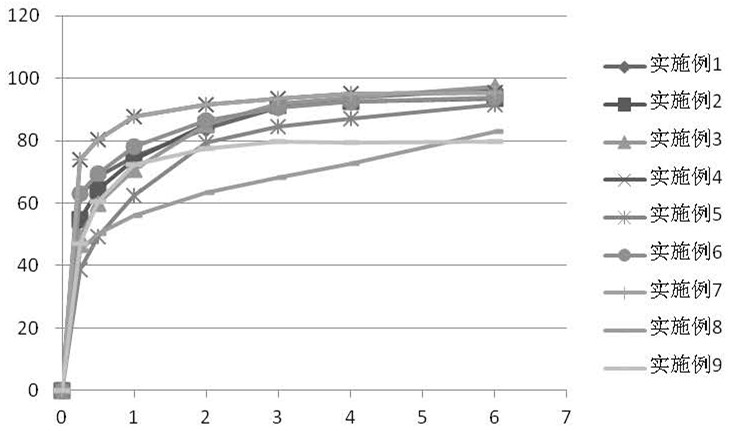
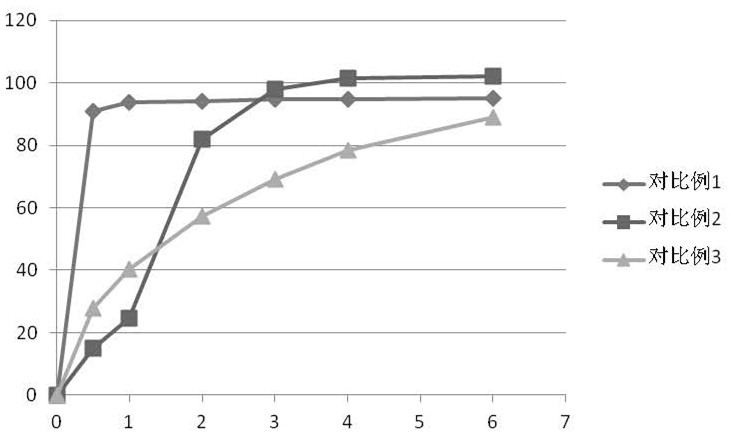
Embodiment 1
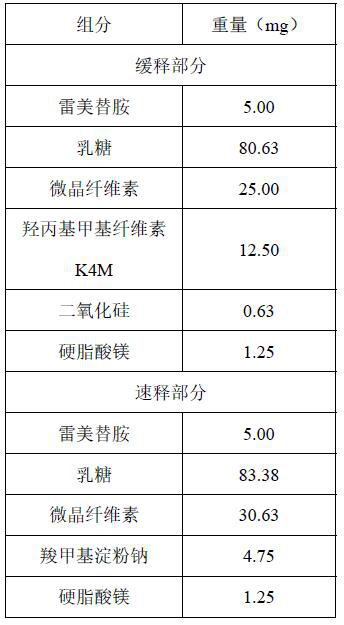
[0093] This embodiment provides a rapid-release sustained-release dual-release formulation of ramelteon, the specific prescription is as follows
[0094]
[0095] Concrete preparation method is as follows:
[0096] Mix ramelteon, lactose, microcrystalline cellulose PH102, hydroxypropyl methylcellulose K4M, and silicon dioxide evenly. Then add magnesium stearate and mix well, as the sustained release part.
[0097] Mix ramelteon, lactose, microcrystalline cellulose PH102, and croscarmellose sodium evenly. Then add magnesium stearate and mix well, as the immediate release part.
[0098] The sustained-release part and the immediate-release part of ramelteon were compressed into tablets using a double-layer tablet press, and the filling amount of the two parts of granules was adjusted. The sustained-release part was 100 mg, and the immediate-release part was 150 mg, and the double-layer tablet was pressed.
Embodiment 2
[0100] The present embodiment provides a rapid-release sustained-release dual-release preparation of ramelteon, and the specific prescription is as follows:
[0101]
[0102] Concrete preparation method is as follows:
[0103] Mix ramelteon, lactose, microcrystalline cellulose PH102, hydroxypropyl methylcellulose K4M, and silicon dioxide evenly. Then add magnesium stearate and mix well, as the sustained release part.
[0104] Mix ramelteon, lactose, microcrystalline cellulose PH102, and croscarmellose sodium evenly. Then add magnesium stearate and mix well, as the immediate release part.
[0105] The sustained-release part and the immediate-release part of ramelteon were compressed into tablets using a double-layer tablet press, and the filling amount of the two parts of granules was adjusted. The sustained-release part was 125 mg, and the immediate-release part was 125 mg, and the double-layer tablet was pressed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com