Alkynyl hydroxypropyl cellulose and preparation method and application of temperature-sensitive hydrogel of alkynyl hydroxypropyl cellulose
A temperature-sensitive hydrogel, alkynyl hydroxypropyl technology, applied in the field of biomedical functional materials, can solve the problems of poor temperature sensitivity of hydrogel, poor mechanical strength of biocompatibility, poor automatic degradation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
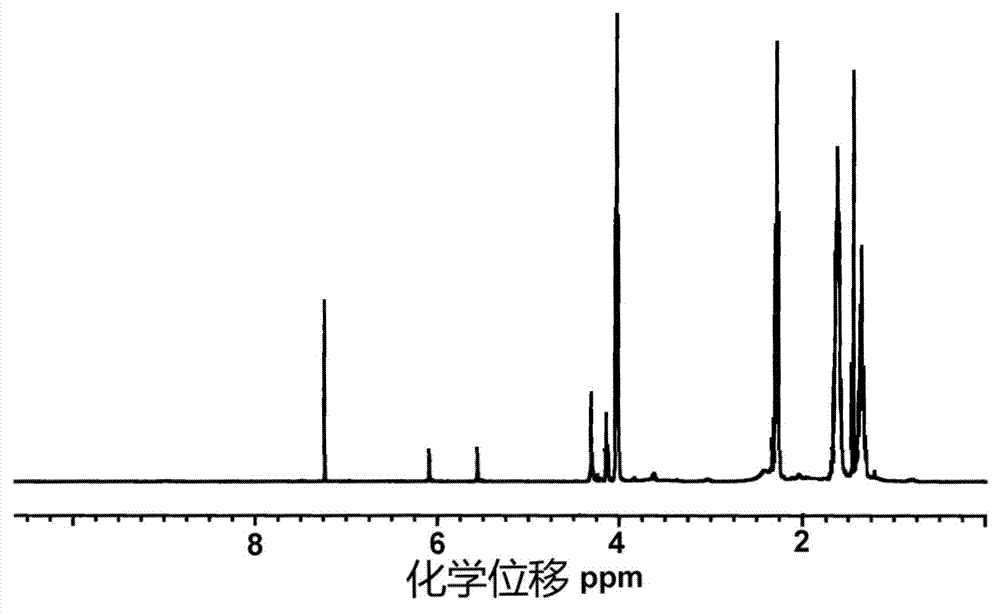
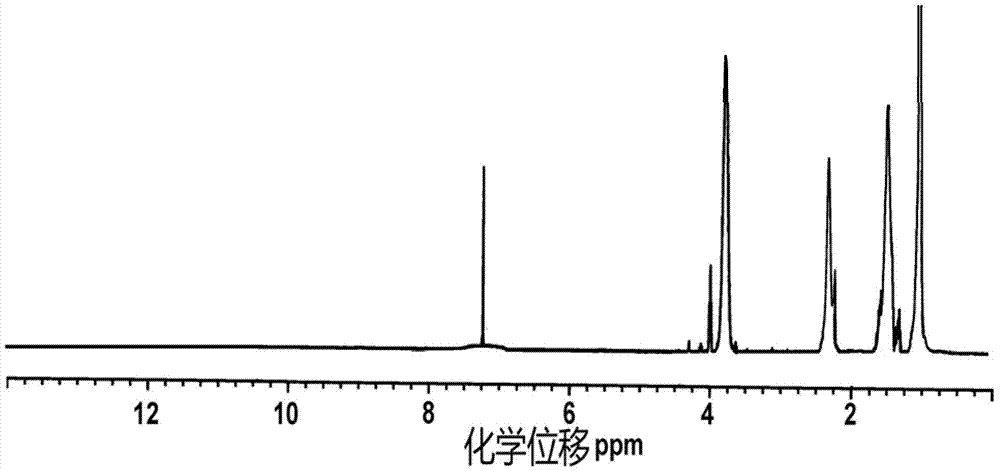
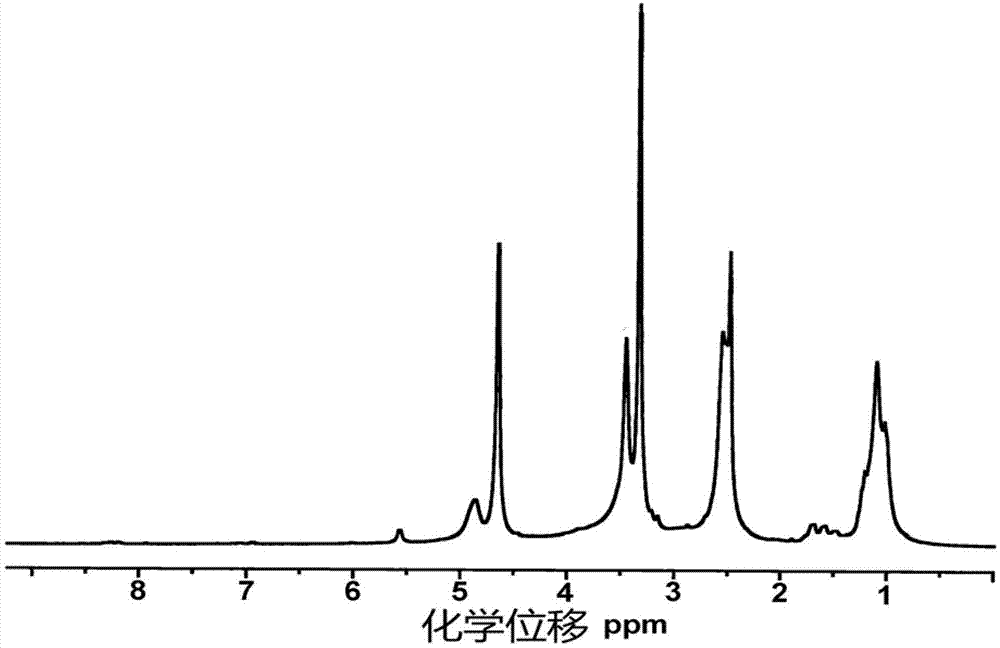
Image
Examples
Embodiment 1
[0074] (1) Add 8gε-caprolactone, 5g hydroxyethyl methacrylate, 0.1g stannous octoate and 0.05g p-hydroxyanisole into the round bottom flask, pump the reactor to vacuum, seal it, and react at 110°C 3h, the product 1 was obtained.
[0075] (2) Dissolve the product 1 obtained in step (1) in 5ml tetrahydrofuran (analytically pure) to make a solution, pour this solution into 50ml methanol to precipitate a white precipitate, repeat the dissolution-precipitation process 4 times, and place the precipitate in vacuum at room temperature Product 2 was obtained after drying for 12 h.
[0076] (3) Dissolve the product 2 obtained in step (2) and 1ml of triethylamine in 15ml of dichloromethane, add 2g of dibromoisobutyryl bromide dropwise at -15°C, and react at 30°C after the addition is complete After 10 h, wash with 100 ml of saturated sodium carbonate solution for 4 times, and then with 100 ml of deionized water for 4 times, then pour the reactant into 250 ml of petroleum ether for 90 mi...
Embodiment 2
[0082] (1) Add 12gε-caprolactone, 2g hydroxyethyl methacrylate, 0.1g stannous octoate and 0.08g p-hydroxyanisole into the reactor, pump the reactor to vacuum, seal it at 130°C and react for 5h to obtain Product 1.
[0083] (2) Dissolve the product 1 obtained in step (1) in 10ml tetrahydrofuran (analytically pure) to obtain a solution, pour this solution into 100ml methanol to precipitate a white precipitate, repeat this dissolution-precipitation process 4 times, and place the precipitate at room temperature Product 2 was obtained after vacuum drying for 24 h.
[0084] (3) Dissolve the product 2 and 3ml of triethylamine obtained in step (2) in 5ml of dichloromethane, add 2.5g of dibromoisobutyryl bromide dropwise at -20°C, after the addition is complete, at 20°C After reacting for 6 hours, wash twice with 50ml saturated sodium carbonate solution, then wash twice with 50ml deionized water, then pour the reactant into 150ml petroleum ether for 30min, and then precipitate the pro...
Embodiment 3
[0090] (1) Add 10gε-caprolactone, 2g hydroxyethyl methacrylate, 0.05g stannous octoate and 0.05g p-hydroxyanisole into the reactor, pump the reactor to vacuum, seal it, and react at 120°C for 3.5 h to give product 1.
[0091] (2) Dissolve the product 1 obtained in step (1) in 5ml tetrahydrofuran (analytically pure), pour this solution into 100ml methanol to precipitate a white precipitate, repeat the dissolution-precipitation process twice, and place the precipitate at room temperature under vacuum Product 2 was obtained after drying for 20 h.
[0092] (3) Dissolve the product 2 and 3ml of triethylamine obtained in step (2) in 15ml of dichloromethane, add 1ml of dibromoisobutyryl bromide dropwise at -20°C, and react at 20°C after the addition is complete 6h, then washed twice with 50ml of saturated sodium carbonate solution, then washed twice with 50ml of deionized water, then poured the reactant into 150ml of petroleum ether for 30min to precipitate product 3.
[0093] (4) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com