Pharmaceutical compositions of ramelteon and methods of use thereof
A technology of ramelteon and composition, applied in the field of ramelteon pharmaceutical composition for the treatment of insomnia and jet lag syndrome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
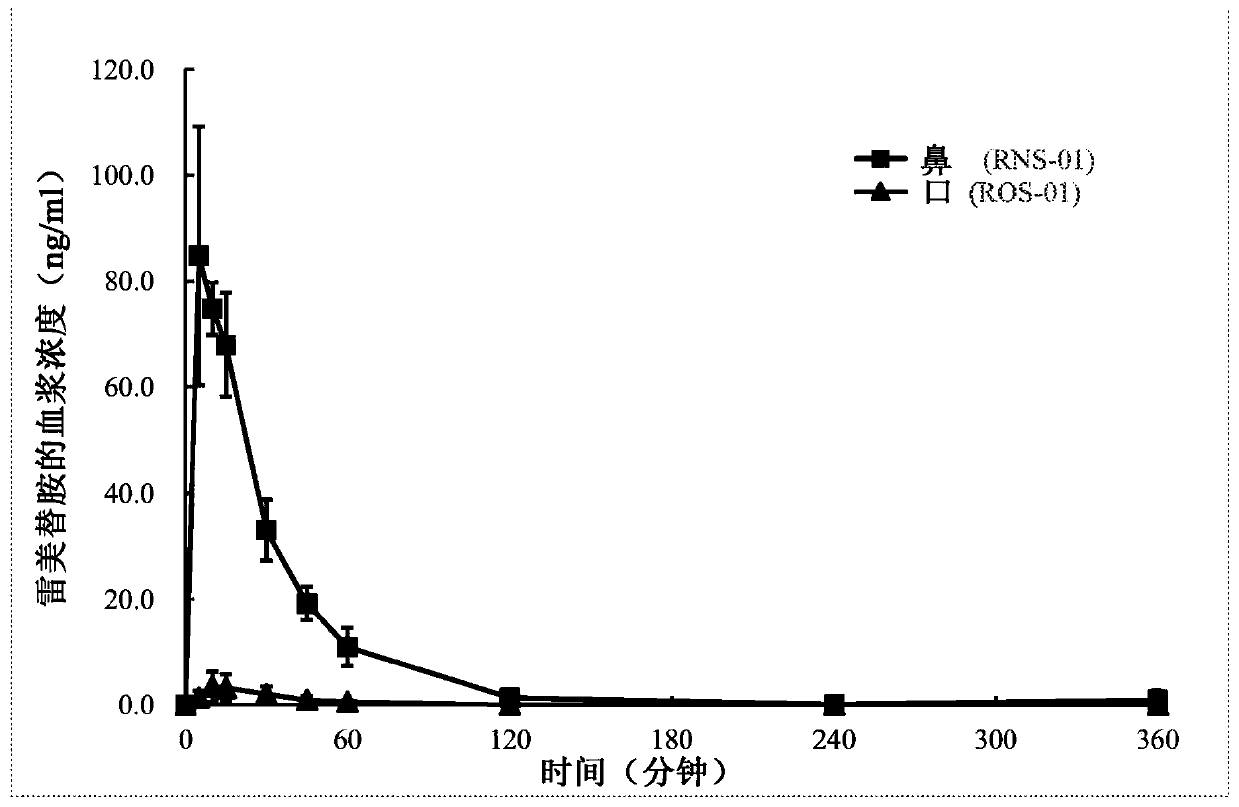
[0024] ramelteon nasal spray
[0025]In one exemplary composition of the present invention, a nasal composition is prepared using a solubilizing agent such as propylene glycol and sulfobutyl ether-beta-cyclodextrin (SBE-CD). First, 5 g of ramelteon was completely dissolved in 150 mL of propylene glycol, then this solution was gradually added to 5 mL of SBE solution (52%, w / v) containing EDTA.2Na and benzalkonium chloride, and finally an appropriate amount of purified water was added to 1L.
[0026]
[0027] After preparation, the drug solution was filtered through a 0.22 μm filter membrane, and then filled into a glass bottle equipped with a metered dose spray pump for intranasal administration at a volume of 0.10 ml per spray. Each spray will deliver 0.5 mg ramelteon intranasally.
Embodiment 2
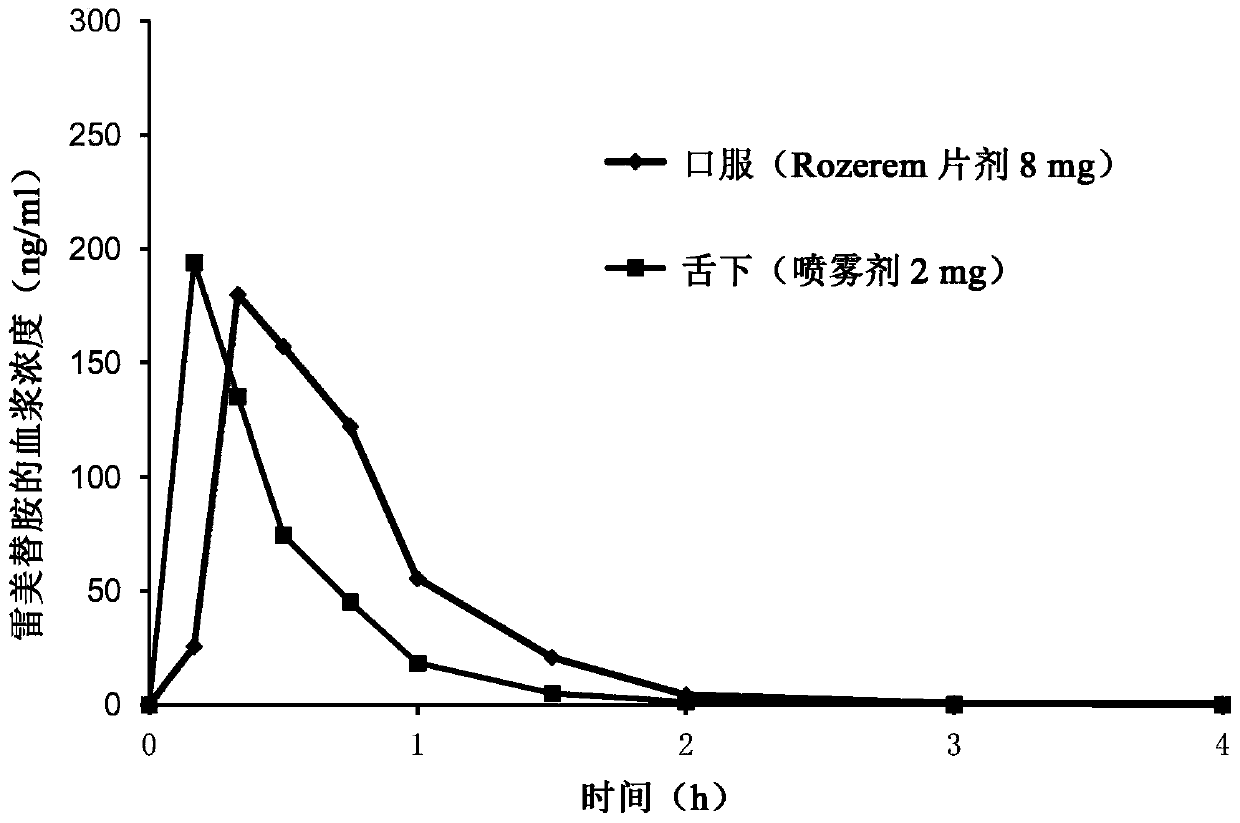
[0029] ramelteon sublingual tablet
[0030] Ramelteon sublingual tablets are prepared by two different methods: direct compression and wet granulation. For direct compression, ramelteon is mixed with excipients. The physical mixture was directly compressed with a single punch tablet press (Shanghai Tianfan Pharmaceutical Machinery Factory, model 6A). Wet granulation methods are listed below:
[0031] 1. Weigh the ingredients according to the recipe.
[0032] 2. Dissolve ramelteon and HP-β-cyclodextrin in ethanol under stirring.
[0033] 3. Mix ramelteon-cyclodextrin solution with CL-SF was mixed for granulation. Pass the wet material through a 40 mesh screen.
[0034] 4. Drying: The wet granules were dried in an oven at 60° C. for 120 minutes.
[0035] 5. Dry sieving: The dry granules were ground and sieved through a 60-mesh sieve.
[0036] 6. Final mixing: the extragranular ingredients ( EASYtab SP and magnesium stearate) are added to the dry granules. The final g...
Embodiment 3
[0040] Dissolution test of ramelteon sublingual tablet
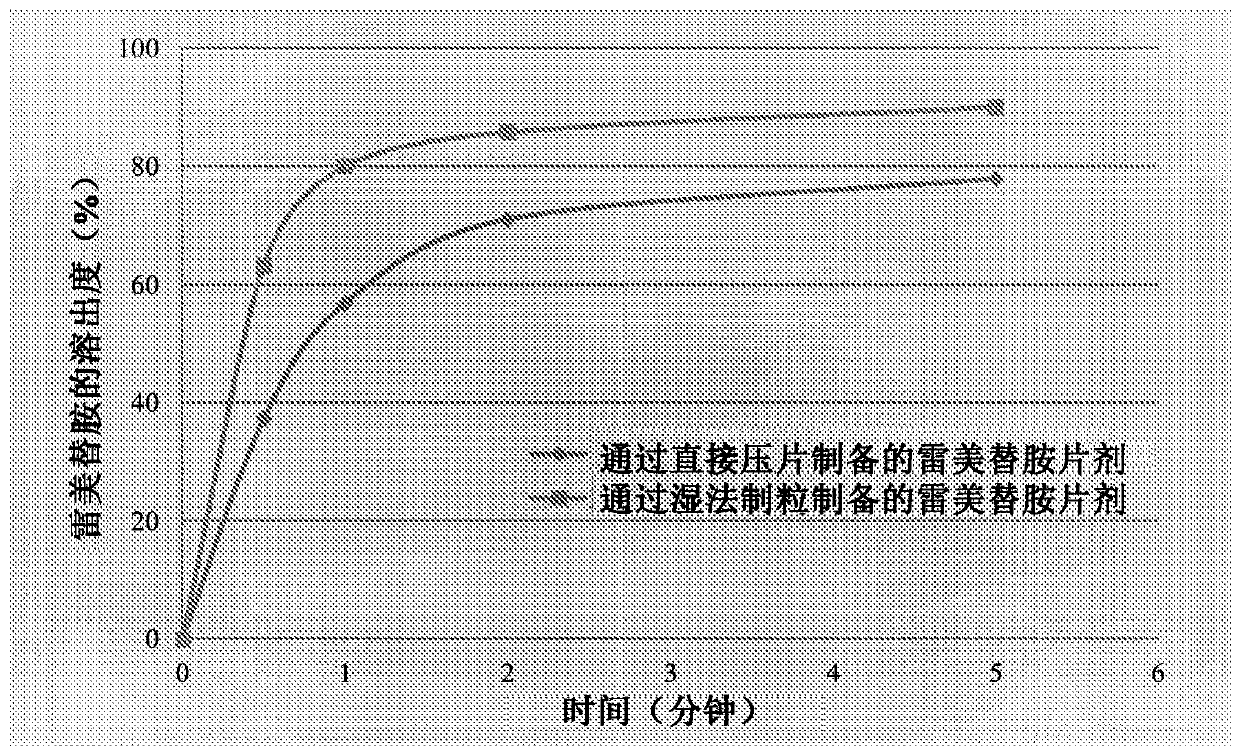
[0041] Dissolution tests were performed according to USP 36 / NF 31 Dissolution Test Apparatus II (paddle method) using a dissolution tester (TIANDA TIANFA - Manufacturer of Pharmaceutical Testing Instruments, ZRS-8L). At 37°C, 100 mL of purified water was used as the dissolution medium. The paddle speed was maintained at 50 rpm. 2 mL aliquots were withdrawn at regular intervals and the sample volume was replaced with an equal volume of purified water. Dissolution samples were analyzed by HPLC. The dissolution profiles of ramelteon tablets prepared by direct compression and wet granulation were as follows: figure 1 As shown, the dissolution rate of the wet granulation tablet was faster than that of the tablet prepared by direct compression.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com