Heparin pharmaceutical composition, nasal spray and preparation method and application of heparin pharmaceutical composition or nasal spray
A composition and heparin technology, applied in the field of medicine, can solve problems such as poor, single prevention effect of new coronavirus infection, and achieve the effect of enhancing protection ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1. Materials
[0039] Unfractionated heparin sodium was purchased from Ron (185 USP units / mg), Arbidol (purity 98%, purchased from Aladdin)
[0040] 293T cells overexpressing ACE2 and the pseudovirus of SARS-Cov-2 S protein were purchased from Changsha Junyi Huaxiang Biotechnology Co., Ltd.
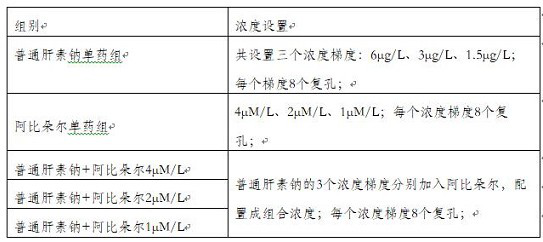
[0041] In this embodiment, the concentration gradient of the combined drug is set as follows:
[0042] Unfractionated heparin sodium: 6μg / L, 3μg / L, 1.5μg / L;
[0043] Arbidol: 4μM / L, 2μM / L, 1μM / L
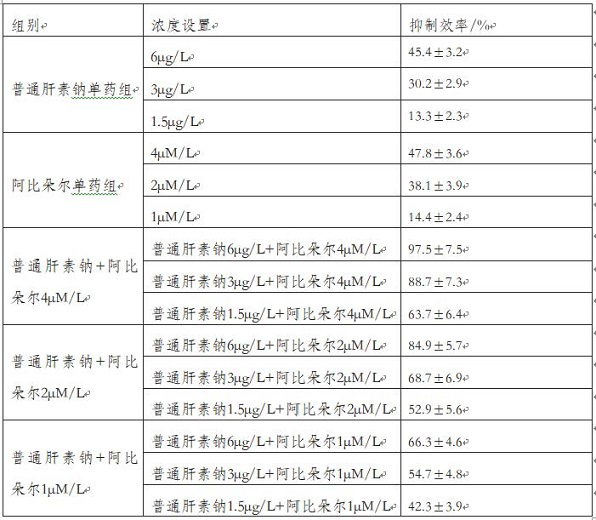
[0044] In this embodiment, the formulas with the concentrations shown in Table 1 are configured altogether.
[0045] Table 1
[0046]
[0047] 2. Experimental method
[0048] Mix 200 TCID50 of a pseudovirus expressing SARS-CoV-2 S protein with a series of drug combinations of different concentrations, with a total volume of 100 μl; add to a 96-well cell culture plate, incubate at 37°C for 1 hour, add 100 μl containing 20,000 overexpressed 293T cells with ACE2; negative control gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com