Tablet containing ramelteon and copovidone
A technology of ramelteon and copovidone, which is applied in the field of solid pharmaceutical compositions, can solve problems such as slow dissolution, failure to meet standard requirements, and influence on bioavailability, and achieve good adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0030] In the following examples and comparative examples, for preparation additives, products meeting the quality standards of Part II of the Chinese Pharmacopoeia 2010 Edition were used.
[0031] Reference Example 1
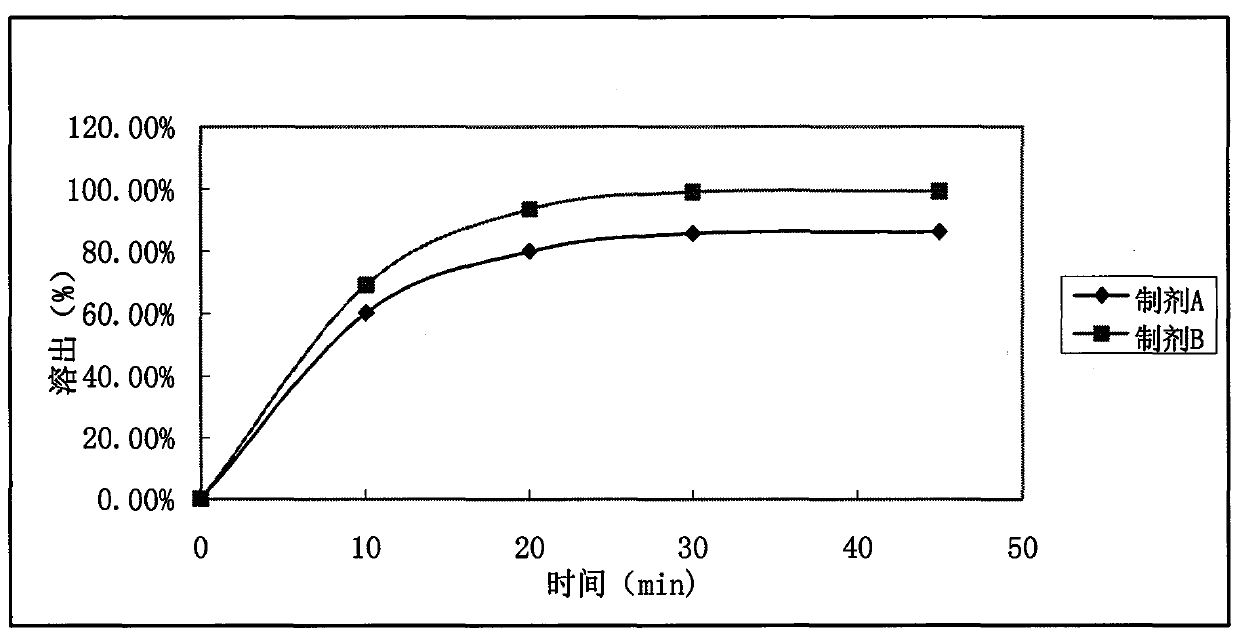
[0032] According to the formula in Table 1, ramelteon was micronized and sieved through a 200-mesh sieve, fully mixed with lactose, corn starch, and hydroxypropylmethyl cellulose, made into granules with water, mixed with magnesium stearate after drying, and pressed tablets, to obtain preparation A; fully mix ramelteon (micronized), lactose, cornstarch, hydroxypropyl methylcellulose and copovidone, make granules with water, add magnesium stearate after drying, and mix evenly. Tablets were obtained to obtain preparation B.
[0033] Table 1
[0034]
[0035] Note: The raw and auxiliary materials in the table are the dosage per 1000 preparation units
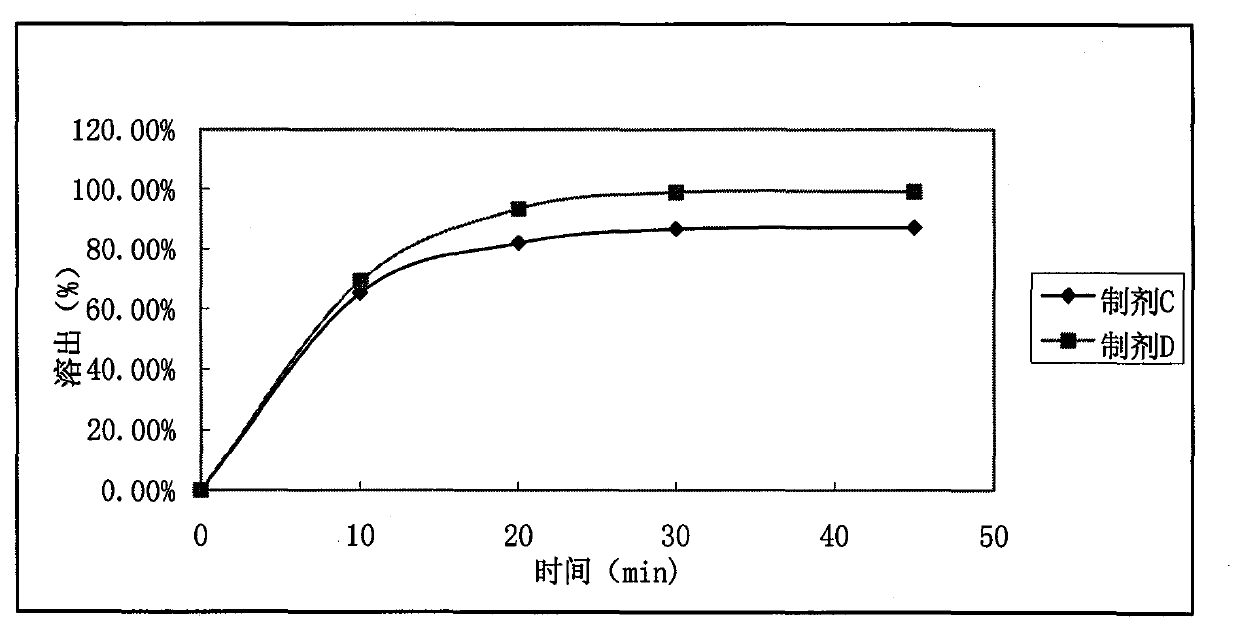
[0036] Reference Example 2
[0037] According to the formula in Table 2, ramelteon was micronized and pas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com