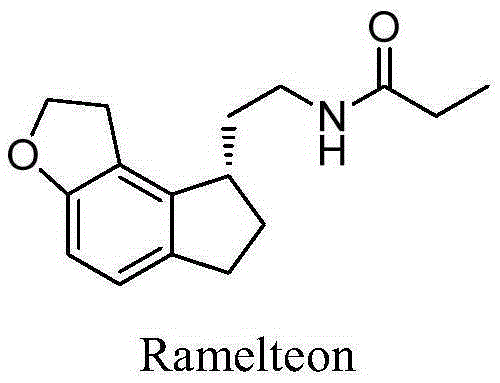
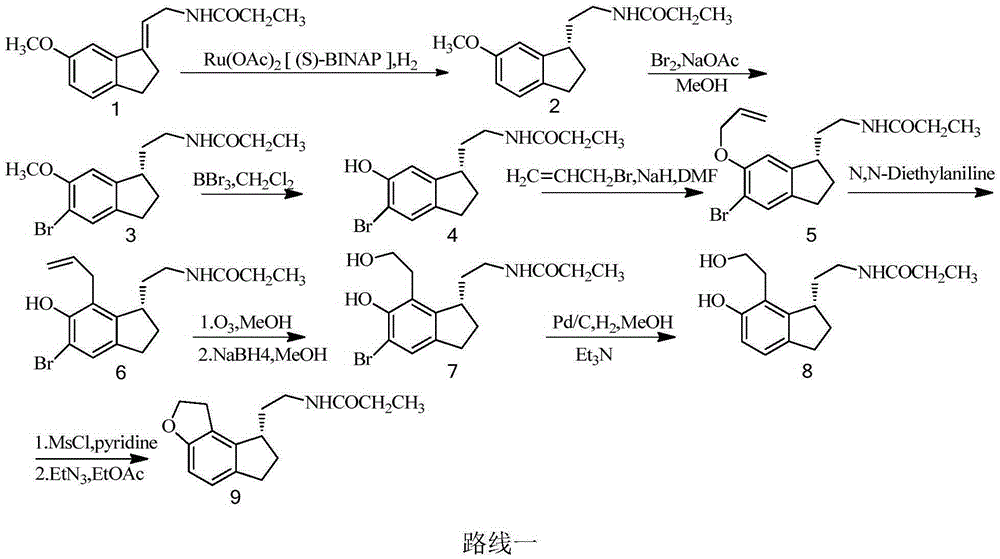
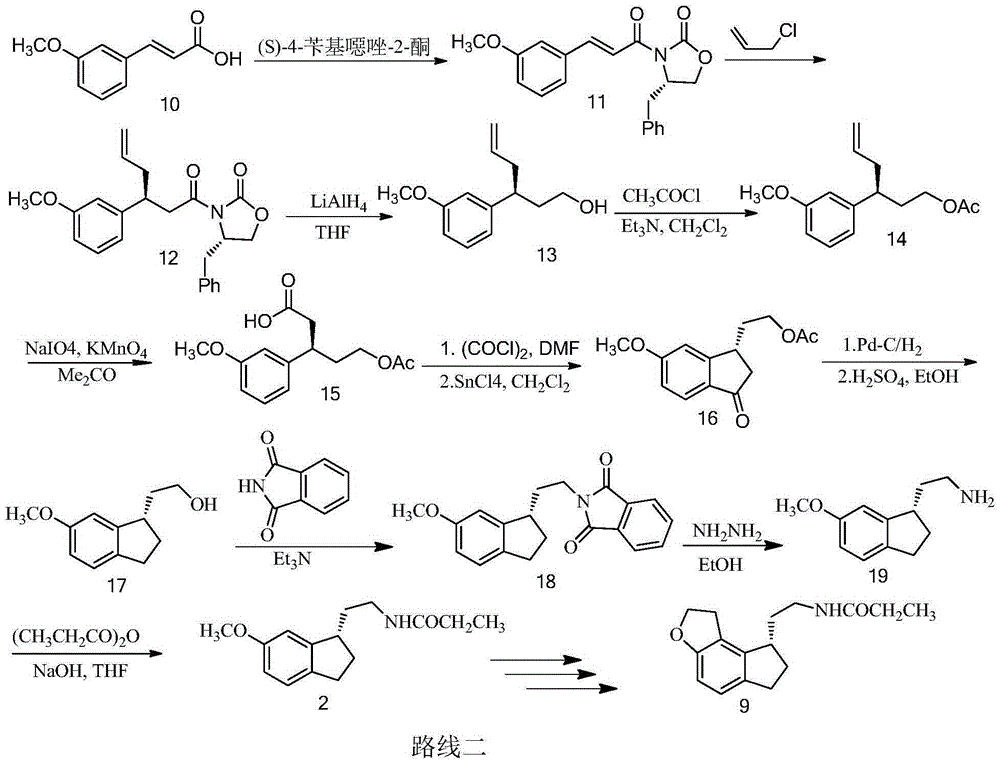
Method for preparing ramelteon intermediate by racemization
A compound and catalyst technology, applied in the field of preparing ramelteon intermediates, can solve the problems of being unsuitable for industrial production, expensive reagents, and high cost, and achieve the effects of environmental protection, simple post-processing, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 : 2-(1,2,6,7- Tetrahydro -8H- Indeno -[5,4,-b] Furan -8- Subunit ) Preparation of acetic acid
[0040]Type R-I (10.0g, 0.046mol) and acetic acid (100ml) were placed in a three-necked flask, stirred and dissolved, and Cu(NO3)2·3H2O (2.214g, 0.009mol) and 65% TBHP (peroxide tert-butanol), 21.2ml, 0.137mol), heated to 70°C, reacted for 8h, TLC detected that the raw material basically disappeared, cooled to room temperature, added the reaction solution into 500ml water, stirred for 2h, extracted with ethyl acetate (100ml×3 times ), combined organic phases, washed with saturated brine for 3 times, dried over anhydrous sodium sulfate, suction filtered, rotary evaporated, and passed through the column to obtain yellow oil II (7.46g, 75%), ESI-MS (m / z): 215[ M-H]+, 1HNMR (400MHz, CDCl3) δ: 3.02~3.04(t, 2H), 3.34(s, 2H), 3.40~3.44(t, 2H), 4.64~4.69(t, 2H), 6.15( s,1H),6.85~6.87(d,1H),7.11~7.13(d,1H)
Embodiment 2
[0041] Example 2 : 2-(1,2,6,7- Tetrahydro -8H- Indeno -[5,4,-b] Furan -8- Subunit ) Preparation of acetic acid
[0042] Type R-I (10.0g, 0.046mol), acetonitrile (90ml) and acetic acid (10ml) were placed in a three-necked flask, stirred and dissolved, then Cu(NO3)2·3H2O (2.214g, 0.009mol) and 65 %TBHP (21.2ml, 0.137mol), heated up to 70°C, reacted for 8h, TLC detected that the raw materials basically disappeared, cooled to room temperature, added the reaction solution to 500ml of water, stirred for 2h, extracted with ethyl acetate (100ml×3 times), The organic phases were combined, washed three times with saturated brine, dried over anhydrous sodium sulfate, filtered with suction, rotary evaporated, and passed through the column to obtain yellow oil II (7.40 g, 75%), ESI-MS (m / z): 215 [M- H]+,1HNMR(400MHz,CDCl3)δ:3.02~3.04(t,2H),3.34(s,2H),3.40~3.44(t,2H),4.64~4.69(t,2H), 6.15(s, 1H), 6.85~6.87(d,1H), 7.11~7.13(d,1H)
Embodiment 3
[0043] Example 3 : 2-(1,2,6,7- Tetrahydro -8H- Indeno -[5,4,-b] Furan -8- Subunit ) Preparation of acetic acid
[0044] Type R-I (10.0g, 0.046mol) and acetic acid (100ml) were dissolved in a three-necked flask, stirred and dissolved, then Cu(NO3)2·3H2O (2.214g, 0.009mol) and 65% TBHP (21.2ml , 0.137mol), heated to 30°C, reacted for 24h, TLC detected that the raw materials basically disappeared, cooled to room temperature, added the reaction solution to 500ml water, stirred for 2h, extracted with ethyl acetate (100ml×3 times), combined the organic phase, saturated Washed with saline for 3 times, dried over anhydrous sodium sulfate, suction filtered, rotary evaporated, passed through the column to obtain yellow oil II (7.34g, 74%), ESI-MS (m / z): 215[M-H]+, 1HNMR (400MHz, CDCl3) δ: 3.02~3.04(t,2H), 3.34(s,2H), 3.40~3.44(t,2H), 4.64~4.69(t,2H), 6.15(s,1H), 6.85~ 6.87(d,1H),7.11~7.13(d,1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com