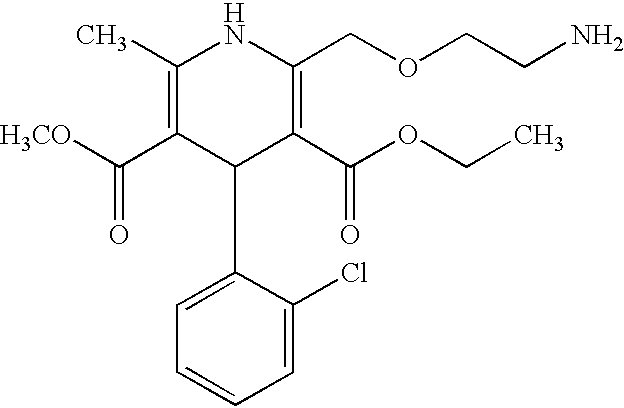
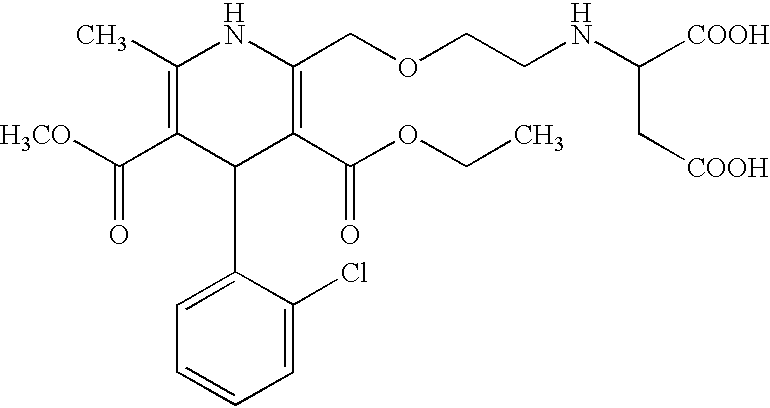
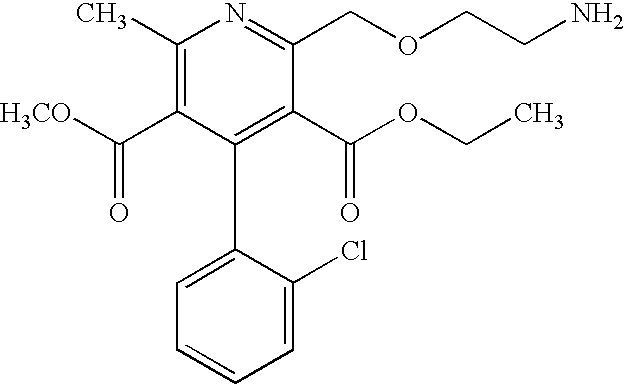
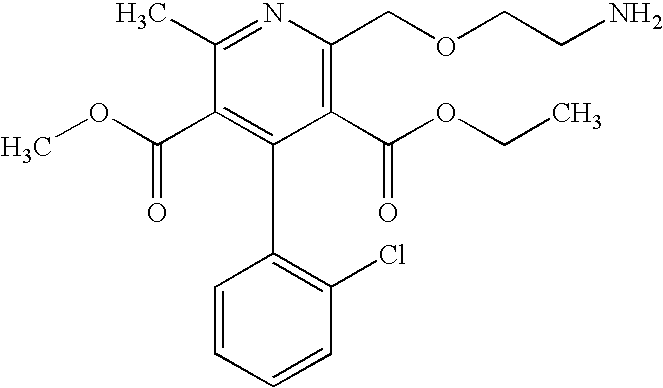
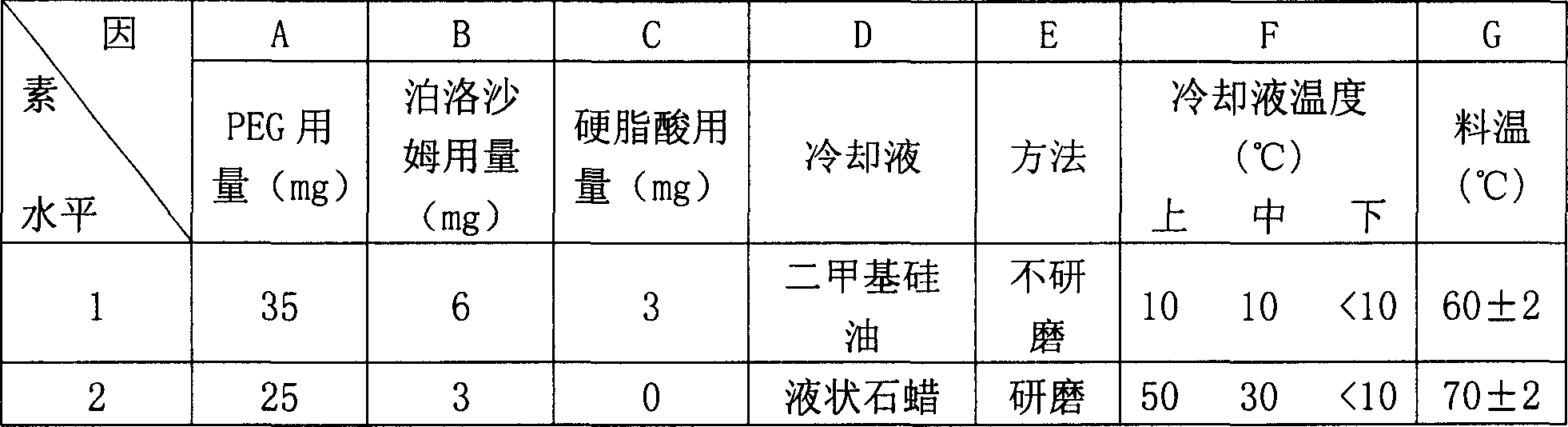
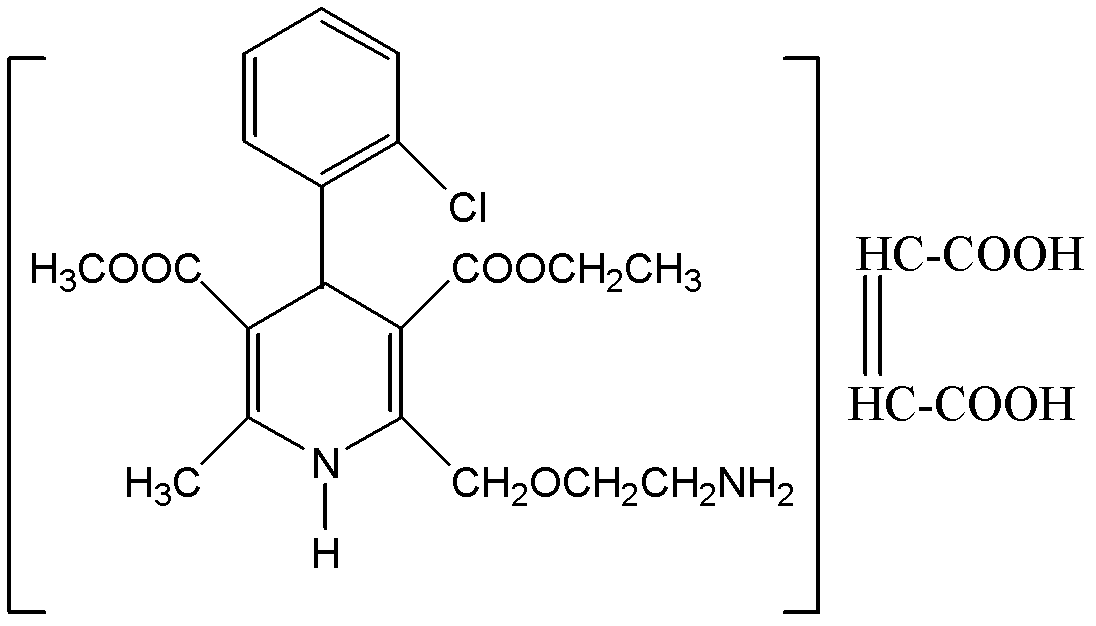
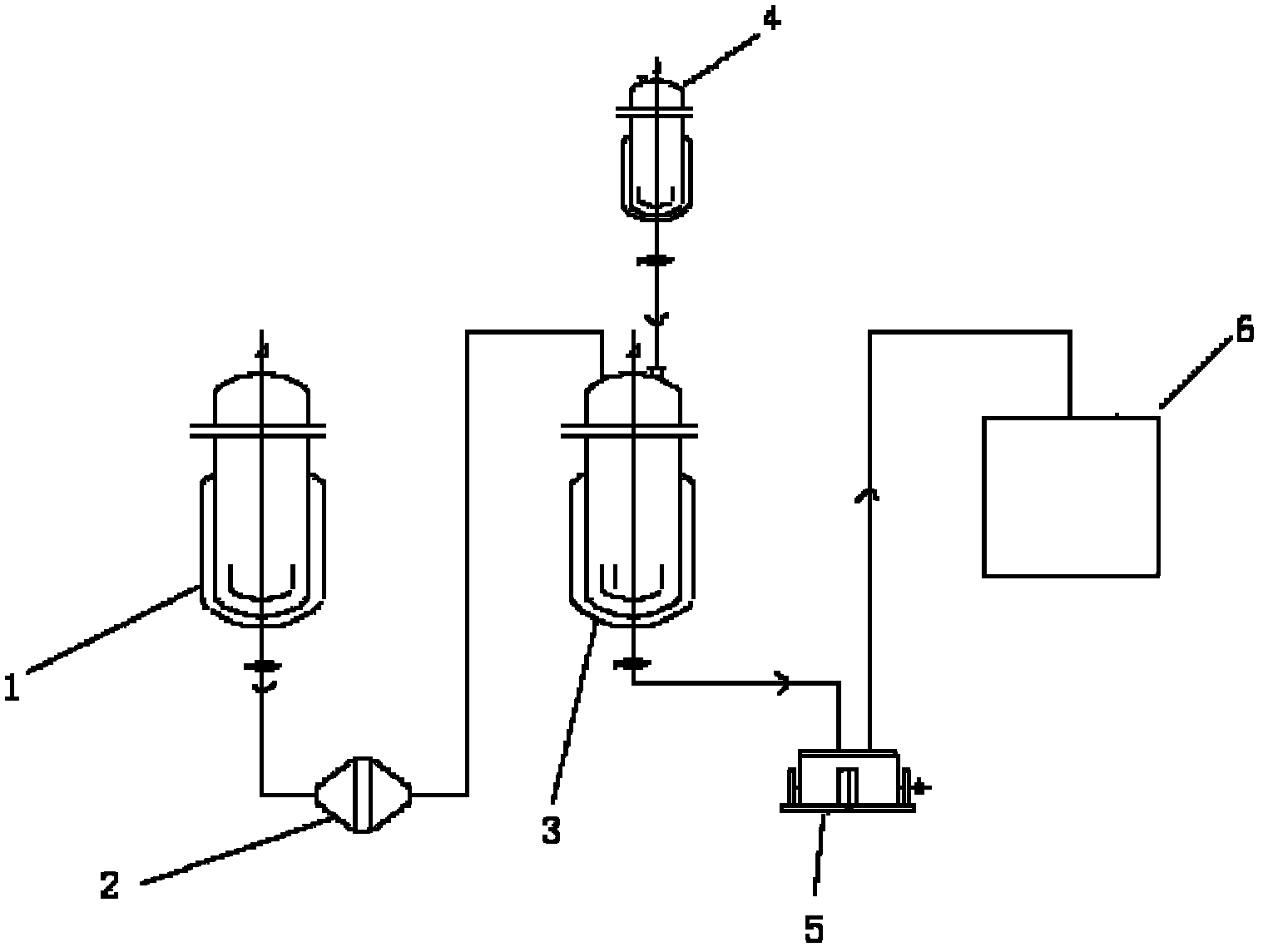
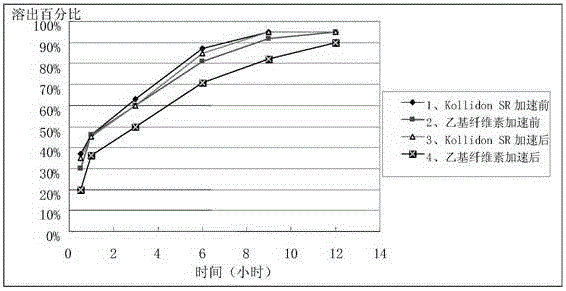
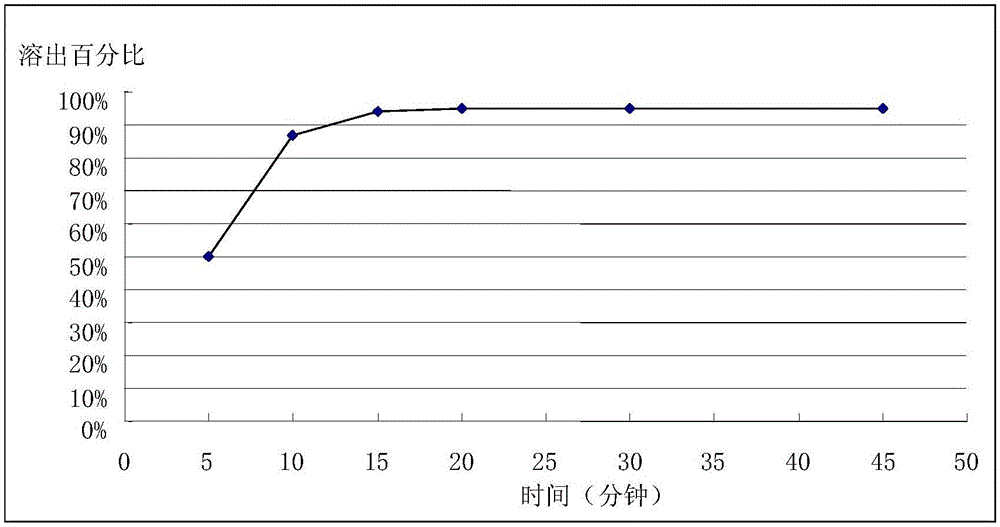
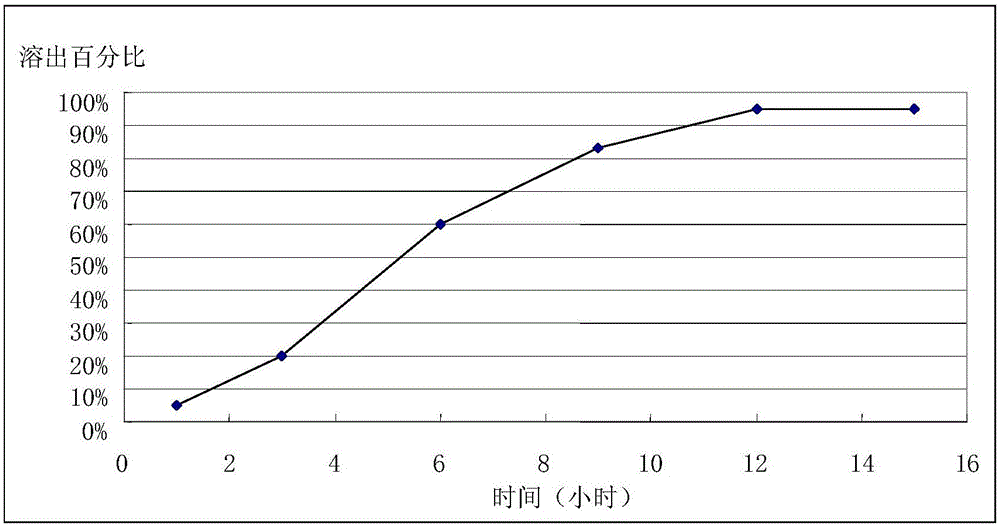
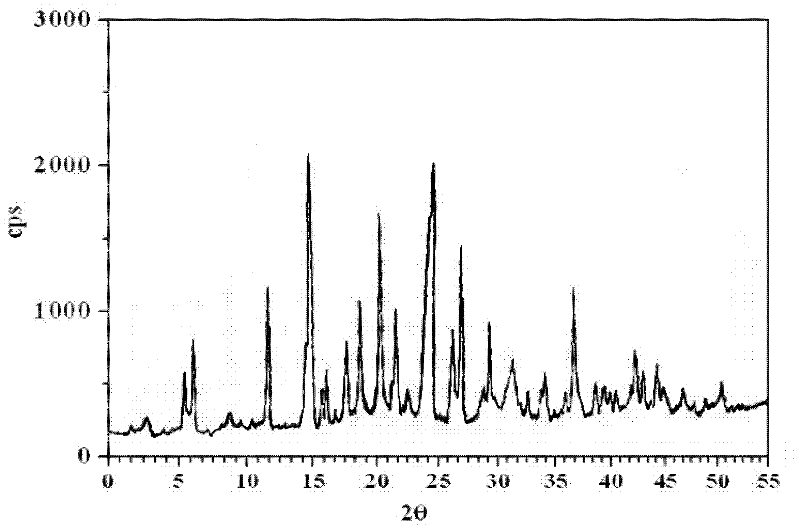
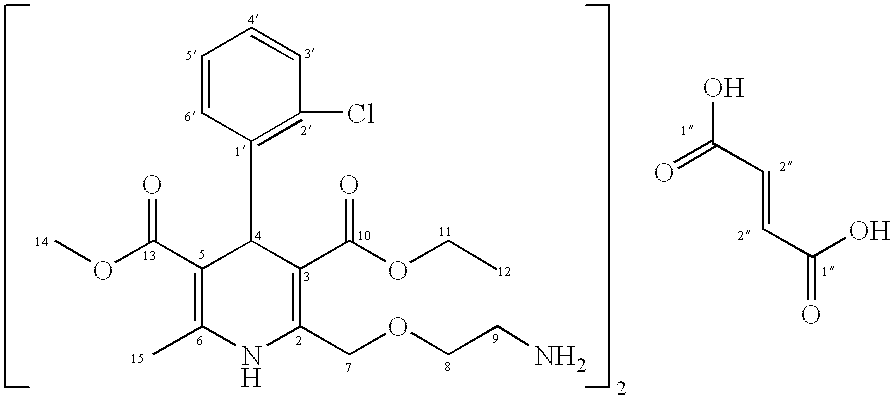
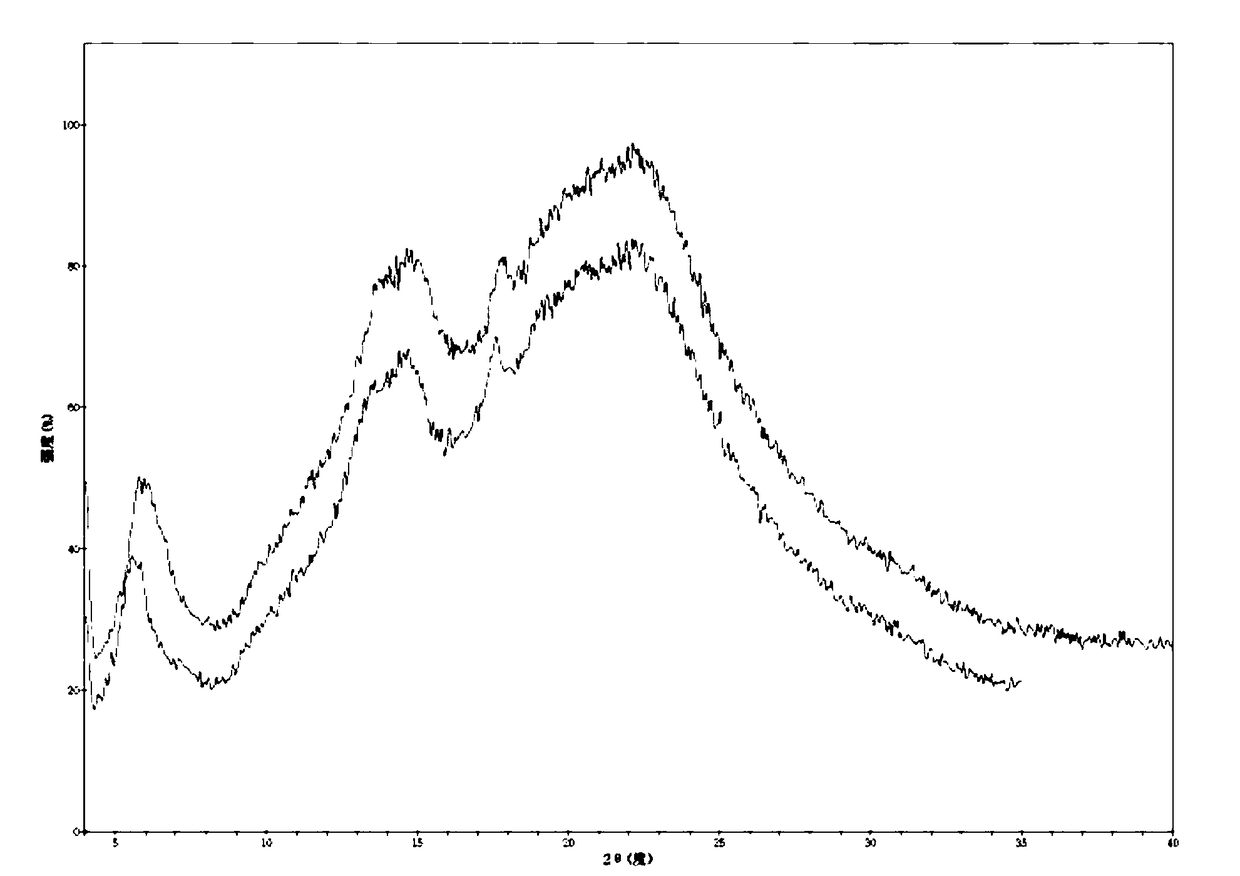
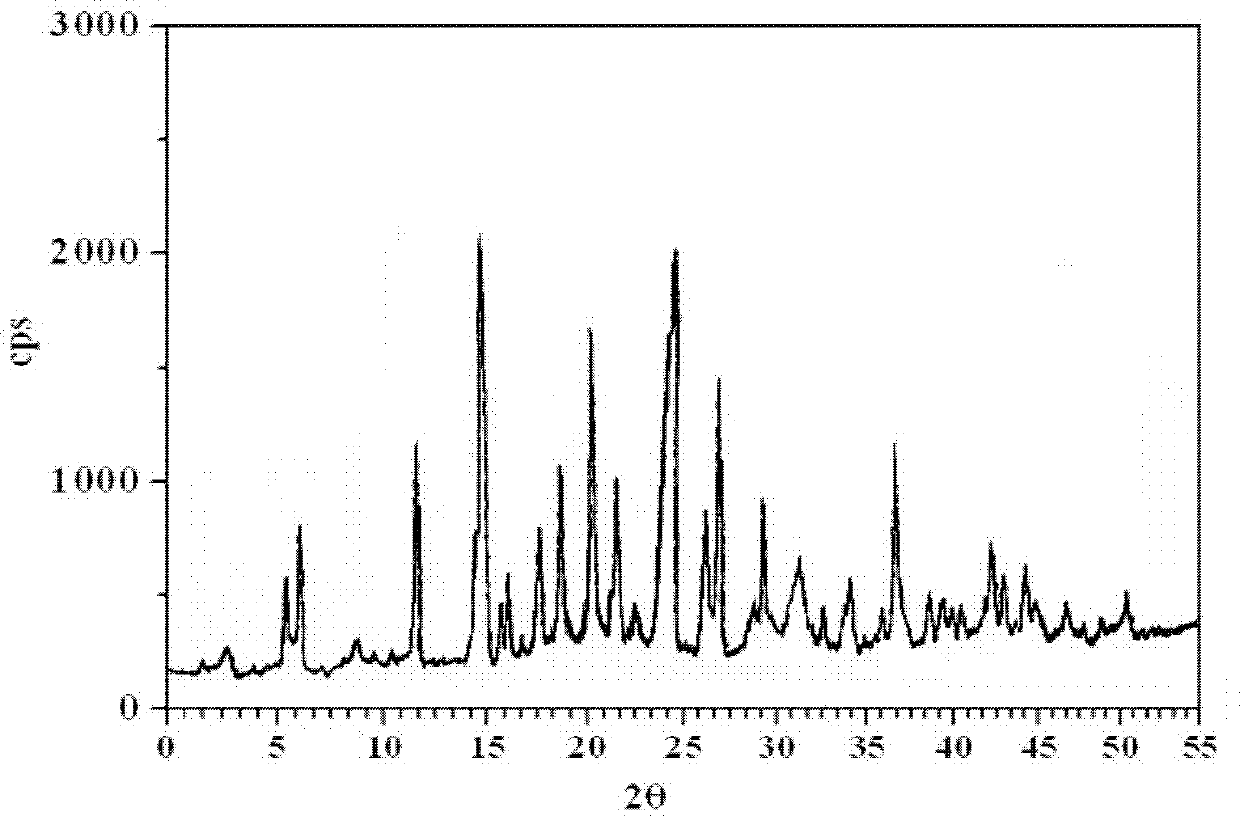
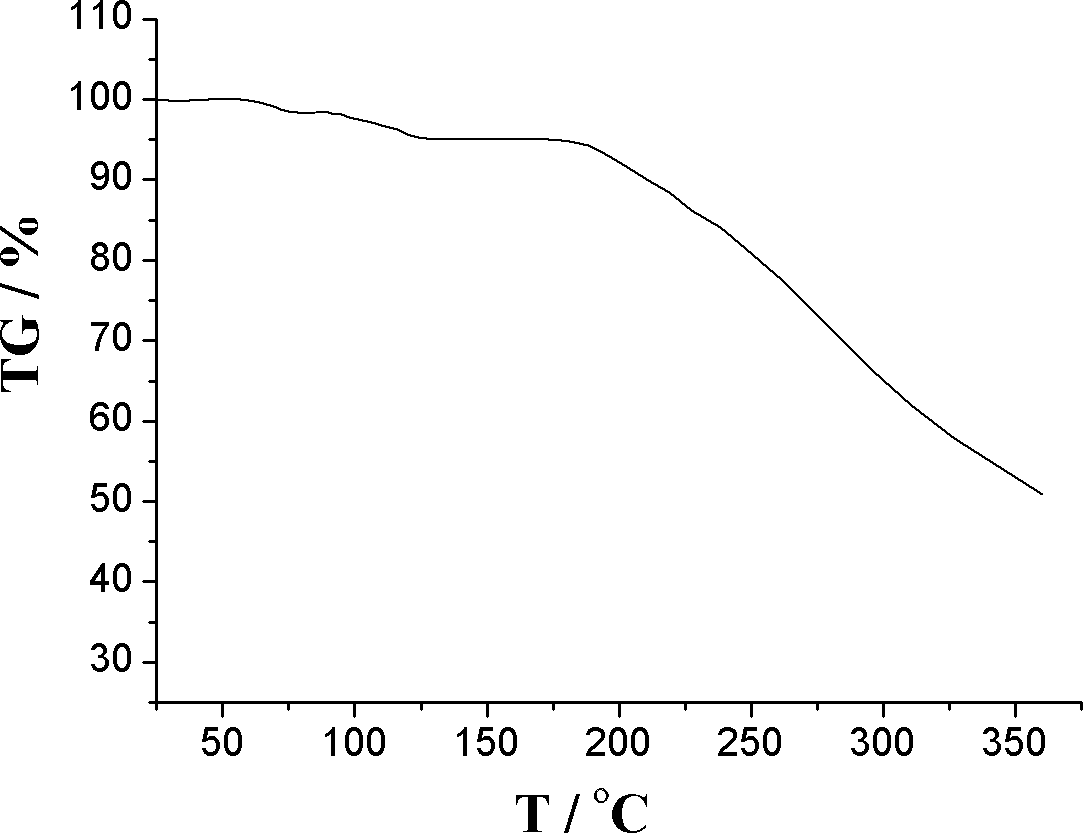
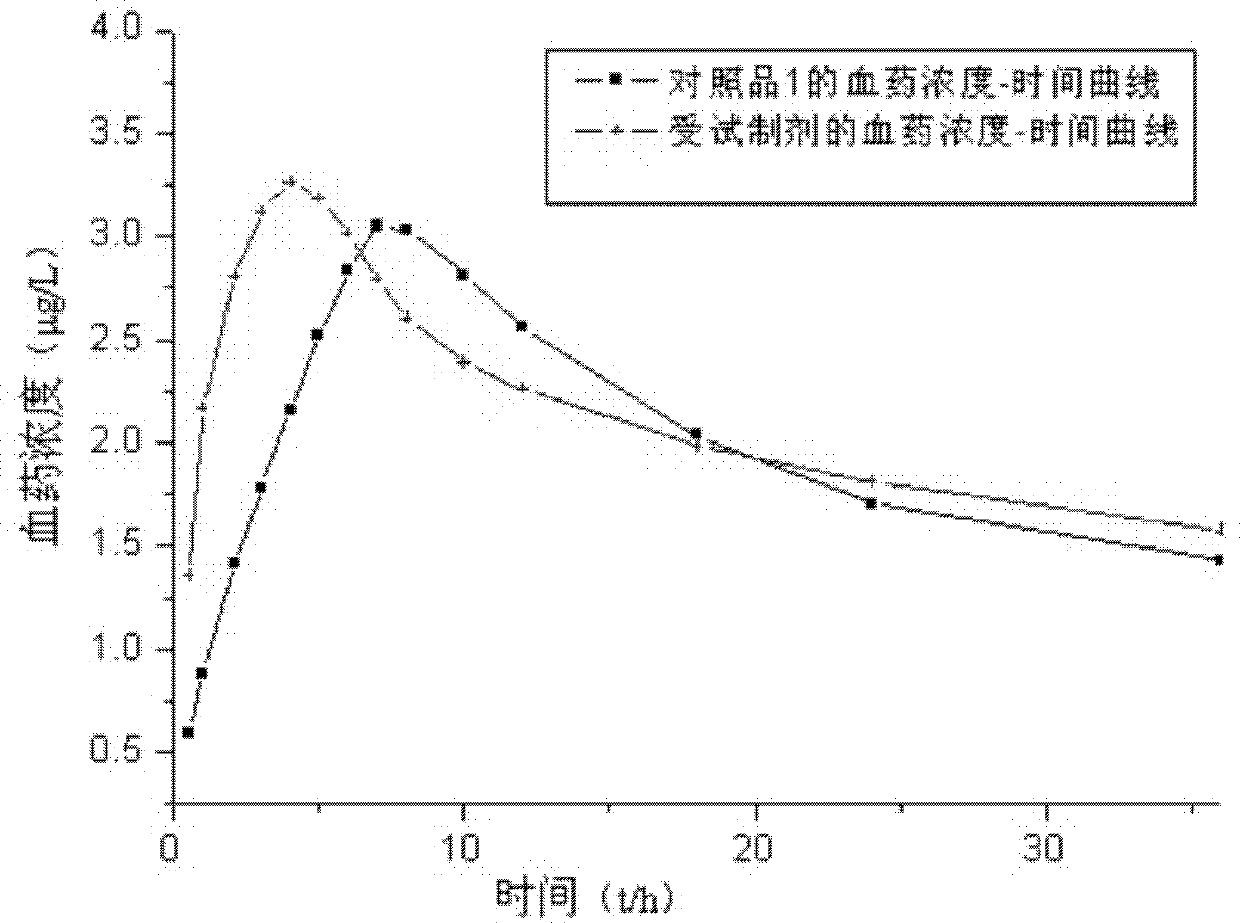
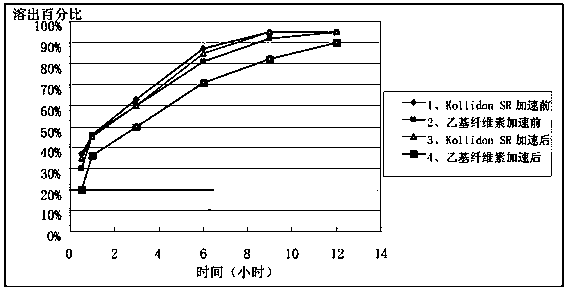
Patents
Literature
36 results about "Amlodipine Maleate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
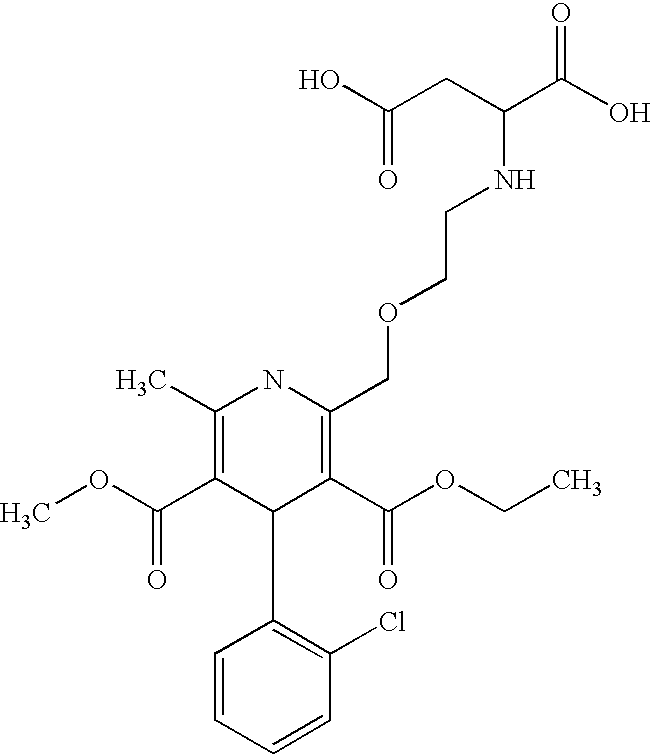
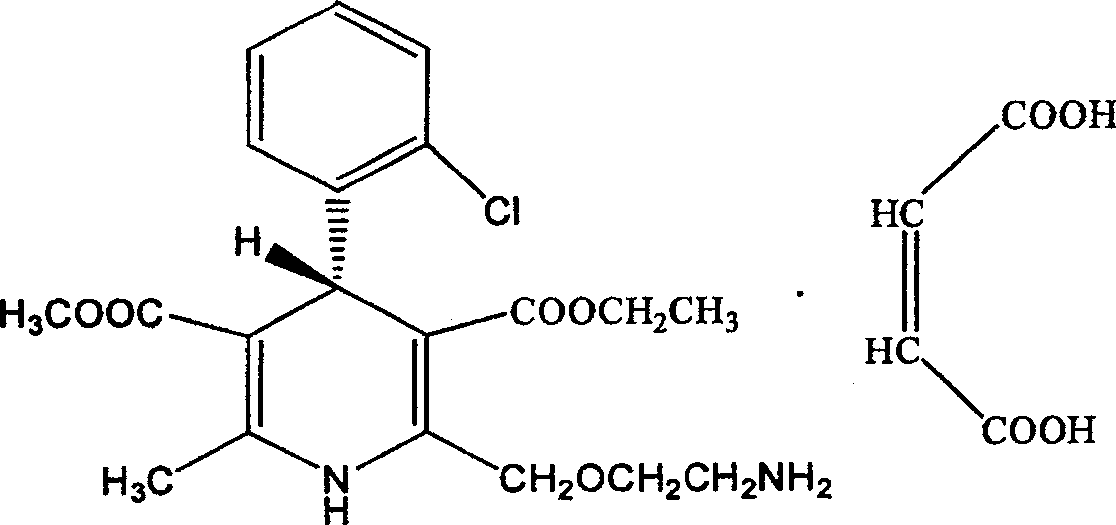
The maleate salt of amlodipine, a synthetic phenylpyridine vasodilator with antihypertensive and antianginal effects. Amlodipine inhibits the influx of extracellular calcium ions into myocardial and peripheral vascular smooth muscle cells, thereby preventing vascular and myocardial contraction. Furthermore, decreased myocardial contractility and dilation of the main coronary and systemic arteries lead to increased blood flow and oxygen delivery to the myocardial tissue and decreases total peripheral resistance. This agent may also modulate multi-drug response activity through inhibition of the p-glycoprotein efflux pump.
Pharmaceutical compositions comprising amlodipine maleate
InactiveUS6919087B2Treating and preventing anginaTreating and preventing and hypertensionEdge grinding machinesBiocideMedicineAmlodipine Maleate
An amlodipine maleate pharmaceutical composition is provided with good stability when formulated with a pH within the range of 5.5 to 7, when measured as a 20 wt % aqueous slurry. The stability can also be aided by making the pharmaceutical composition from amlodipine maleate particles having an average particle size of greater than 20 microns, preferably greater than 100 microns.
Owner:SYNTHON BV
L-amlodipine besilate dripping pill and its preparing method
InactiveCN1899268ASuit one's needsIncrease the dosage form of clinical useOrganic active ingredientsPill deliveryMedicineAmlodipine Maleate
The present invention relates to L-amlodipine besilate dripping pill and its preparation process and belongs to the field of medicine preparing technology. The recipe of the L-amlodipine besilate dripping pill includes L-amlodipine besilate, dripping pill substrate, antioxidant, surfactant, coating material, etc. The preparation process includes the first preparing L-amlodipine besilate into fine powder over 150 mesh, the subsequent melting the substrate, adding fine L-amlodipine besilate powder and supplementary material, mixing to form dispersed liquid, and dropping the dispersed liquid into condensating liquid to form dripping pill with or without coating. Each of the dripping pills contains L-amlodipine besilate in 1.25-15mg and weighs 10-100 mg.
Owner:AOLING BODA MEDICINE SCI & TECH DEV BEIJING
Formulations of amlodipine maleate
InactiveUS20050019395A1Reduce productionCertain stabilityBiocidePill deliveryMedicineAmlodipine Maleate
The present invention provides improved, more stable formulations of amlodipine maleate where the formulations comprise from none to a minimal amount of magnesium. Such stable formulations show decreased production of the impurity amlodipine aspartate. Accordingly, the present invention provides formulations of amlodipine maleate comprising lubricants such as sodium stearyl fumarate, dimeticone, macrogol 6000, hydrogenated castor oil, and stearic acid. Methods of making and using the improved formulations are also provided.
Owner:TEVA PHARM USA INC
Method of resolving amlodipine racemate
The present invention relates to a process for the resolution of racemic amlodipine into enantiomerically enriched components by precipitation with tartaric acid in the presence of a non-aqueous solvent such as N,N'-dimethylacetamide. The molar ratio of tartaric acid:amlodipine is preferably less than 0.25:1.0 and greater than 0.75:1.0.
Owner:SEPACOR INC
Amlodipine and candesartan pharmaceutical composition and preparation method thereof
ActiveCN102342937ALarge specific surface areaIncrease dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsCross-linkCandesartan
The invention relates to an amlodipine and candesartan pharmaceutical composition. The pharmaceutical composition comprises the following components in parts by weight: 2.5-5 parts of amlodipine hydrate crystal, 4-16 parts of candesartan, 5-50 parts of compressible starch, 10-60 parts of microcrystalline cellulose, 15-40 parts of low-substituted hydroxypropyl cellulose, 10-45 parts of cross-linked polyvinyl pyrrolidone and 1-3 parts of magnesium stearate, wherein the amlodipine is amlodipine maleate hydrate crystal with a molecular formula of C24H29ClN2O9.1.5H2O. The pharmaceutical composition has the advantages that: amlodipine maleate has rapid and stable action, and can be stably released within 24 hours; and the pharmaceutical composition has strong synergism, accumulation and complementation effects, and has high bioavailability.
Owner:HAINAN JINRUI PHARMA
Oral disintegration tablet of Amlodipine mesylate maleic acid, and preparation method
InactiveCN1695618AAppropriate hardnessEasy to pack and carryOrganic active ingredientsPill deliveryAmlodipine MaleateDentistry
An oral disintegrating tablet of amlodipine maleate is prepared from amlodipine maleate, filler, disintegrant, effervescent agent, flavouring, lubricant and flowing aid through conventional process.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Amlodipine and olmesartan medoxomil pharmaceutical composition and preparation method thereof
ActiveCN102327265AQuality improvementGood disintegrationOrganic active ingredientsDrageesCross-linkAMLODIPINE/OLMESARTAN
The invention relates to an amlodipine and olmesartan medoxomil pharmaceutical composition and a preparation method thereof. The amlodipine and olmesartan medoxomil pharmaceutical composition comprises the following components in parts by weight: 2.5-10 parts of amlodipine, 20-40 parts of olmesartan medoxomil, 5-50 parts of compressible starch, 10-60 parts of microcrystalline cellulose, 15-40 parts of low-substituted hydroxypropyl cellulose, 10-45 parts of cross-linked polyvinylpyrrolidone, 1-5 parts of essence and 1-3 parts of magnesium stearate, wherein the amlodipine is an amlodipine maleate hydrate crystal. The pharmaceutical composition has the advantages of rapider and stable acting, strong synergism, accumulation and complementation effects, and high bioavailability.
Owner:HAINAN JINRUI PHARMA CO LTD
Drop pills of amlodipine maleate and preparation method thereof
InactiveCN1879621AEvenly dispersedPromote dissolutionOrganic active ingredientsPill deliverySodium stearatePolyethylene glycol
The invention relates to a maleic acid amiloride horizotnal ball and relative preparation, wherein it is formed by active maleic acid amiloride horizontal and relative base; the active component is the maleic acid amiloride horizontal; the base is the carbowax 1500-20000, geoceric acid, sodium stearate, dodecyl sodium sulfate, etc; the ratio between the maleic acid amiloride horizontal and said base is 1:2-10 as g or kg; and the invention also comprises film material whose components are hydroxypropylmethylcellulose at 1.0-1.2g, titania at 0.8-1.0g, talcum powder at 1.0-1.2g, iron oxide yellow at 0.10-0.15g, sorbate-80 at 0.4-0.5g, and 80% alcohol to be 50ml. The invention has simple operation, low cost and high effect.
Owner:TIANNIAN PHARMA HARBIN
Preparation method of amlodipine maleate
Owner:NORTHEAST PHARMA GRP
Method for preparing levamlodipine from racemic amlodipine maleate
ActiveCN101531629ALow toxicityImprove filtering effectOrganic chemistryCardiovascular disorderN dimethylformamideAmlodipine Maleate
The invention relates to a method for removing maleate of amlodipine maleate, which prepares free base of amlodipine from weak inorganic base, DMF and water as solvent by a settling method. The invention relates to a method for preparing levamlodipine from the amlodipine, which removes dextroisomer through salifying precipitation by using mixed solvent of N, N-dimethylformamide and ethanol and D(-)tartaric acid taken as a chiral reagent, and prepares the levamlodipine through precipitation by adding excessive water. The levamlodipine can be used for preparing levamlodipine besylate.
Owner:JIANGSU SIMCERE PHARMA +1
Preparation method of amlodipine besylate tablets
ActiveCN103356493AAppropriate disintegration timeImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsAmlodipine MaleateAmlodipine besilate
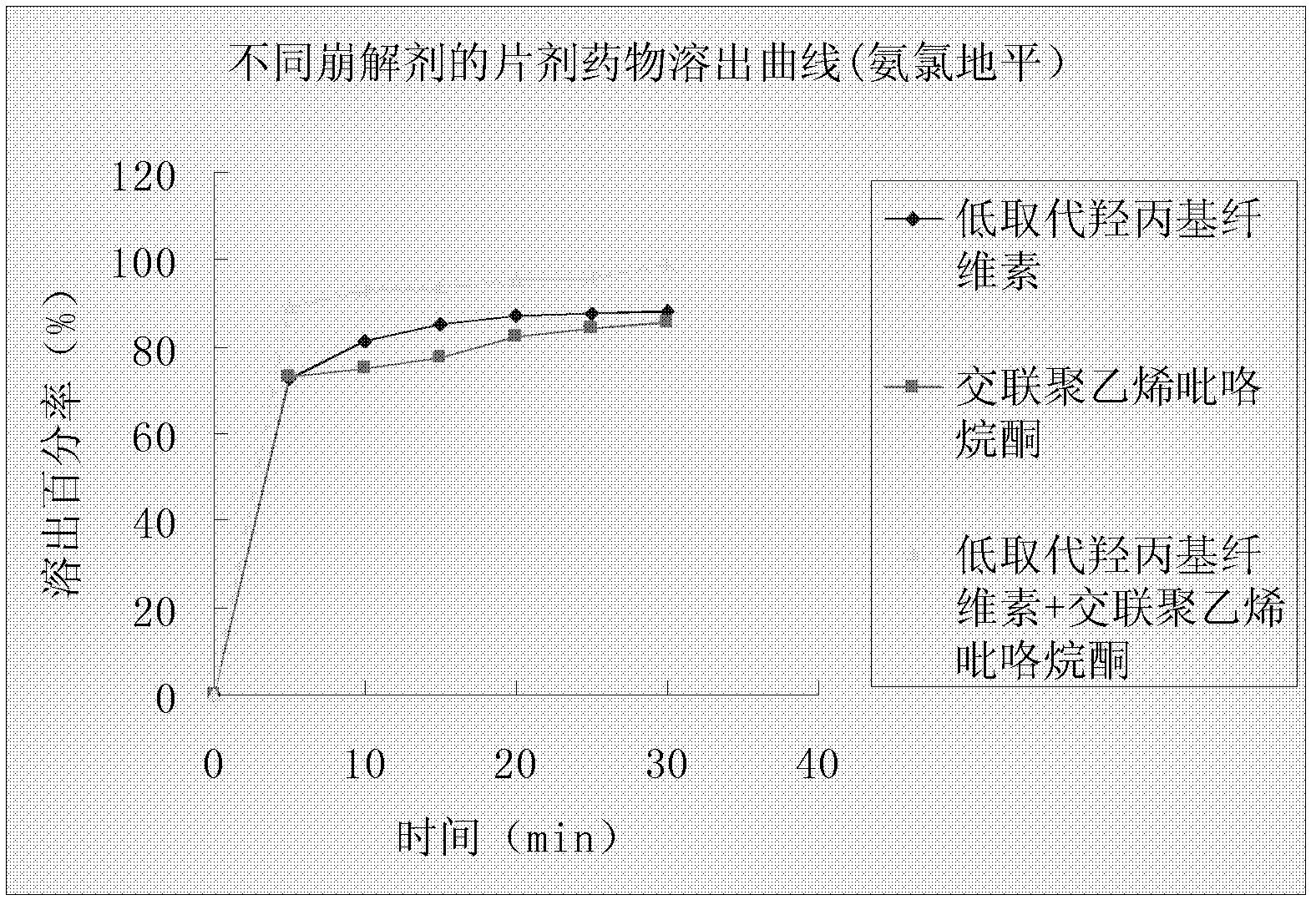
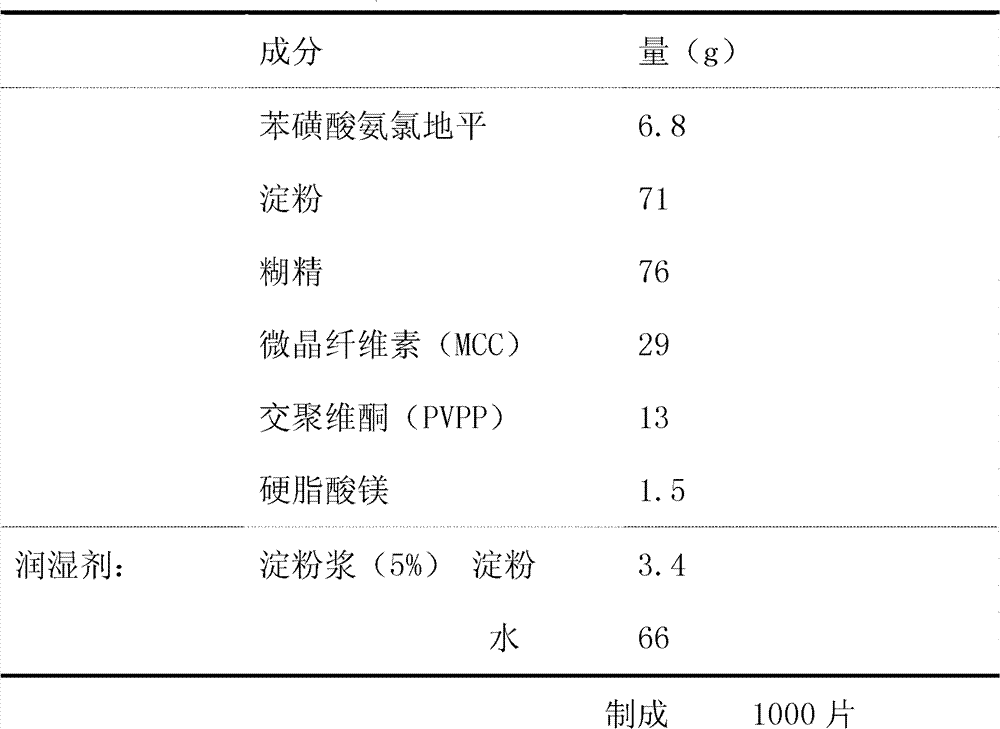
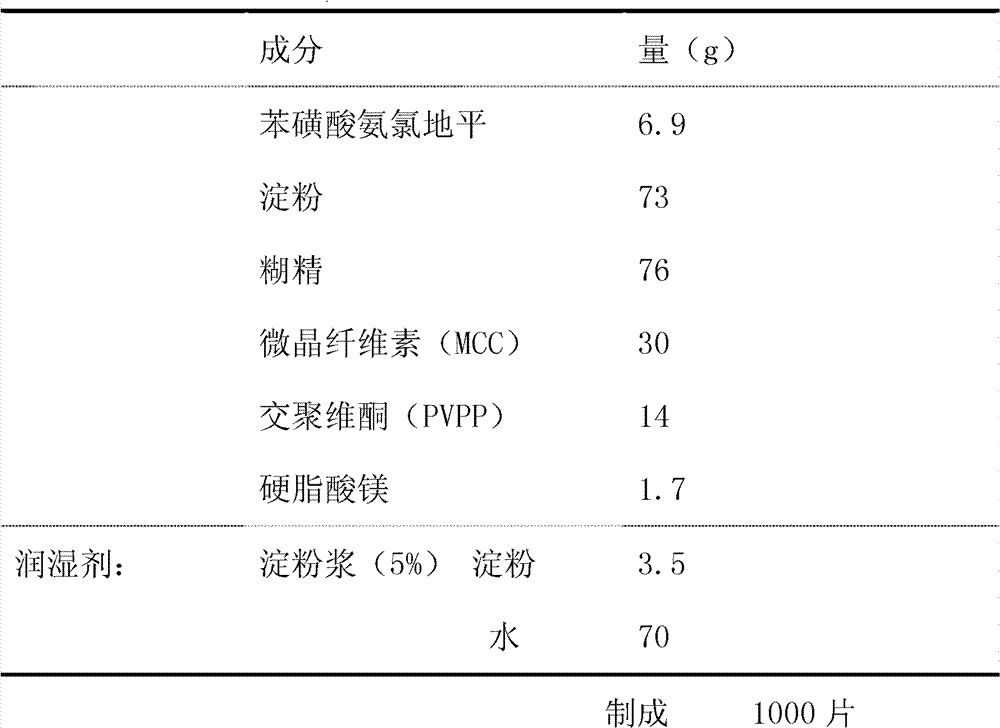
The invention provides a preparation method of amlodipine besylate tablets. The preparation method comprises the following steps of: employing wet granulation and tabletting, filtering each component in a formula through an 80-mesh sieve, and then drying the filtered components at 50 DEG C for future use; evenly mixing amlodipine besylate, microcrystalline cellulose and crospovidone together, forming a soft material from the mixture by using 5% starch slurry, granulating through an 18-mesh sieve, drying at 50 DEG C, and then shaping the granules through the 18-mesh sieve, thereby obtaining granules I; next, evenly mixing starch with dextrin, forming a soft material from the mixture by using 5% starch slurry, granulating through the 18-mesh sieve and drying at 50 DEG C, and then shaping the granules through the 18-mesh sieve, thereby obtaining granules II; finally, mixing the granules I with the granules II, adding magnesium stearate and evenly mixing all the materials, and then tableting the mixture, thus obtaining the amlodipine besylate tablets. According to the invention, the major component amlodipine besylate tablets are white crystalline powder which is good in fluidity; the prepared granules are excellent in both compressibility and fluidity. The disintegration time of the tablets is appropriate, and the content of the tablets, the content uniformity and the dissolution rate all meet the requirements; therefore, the tablets have high clinical application value. The method provided by the invention is simple and suitable for industrial production.
Owner:SHANGHAI SINE PROMOD PHARMA
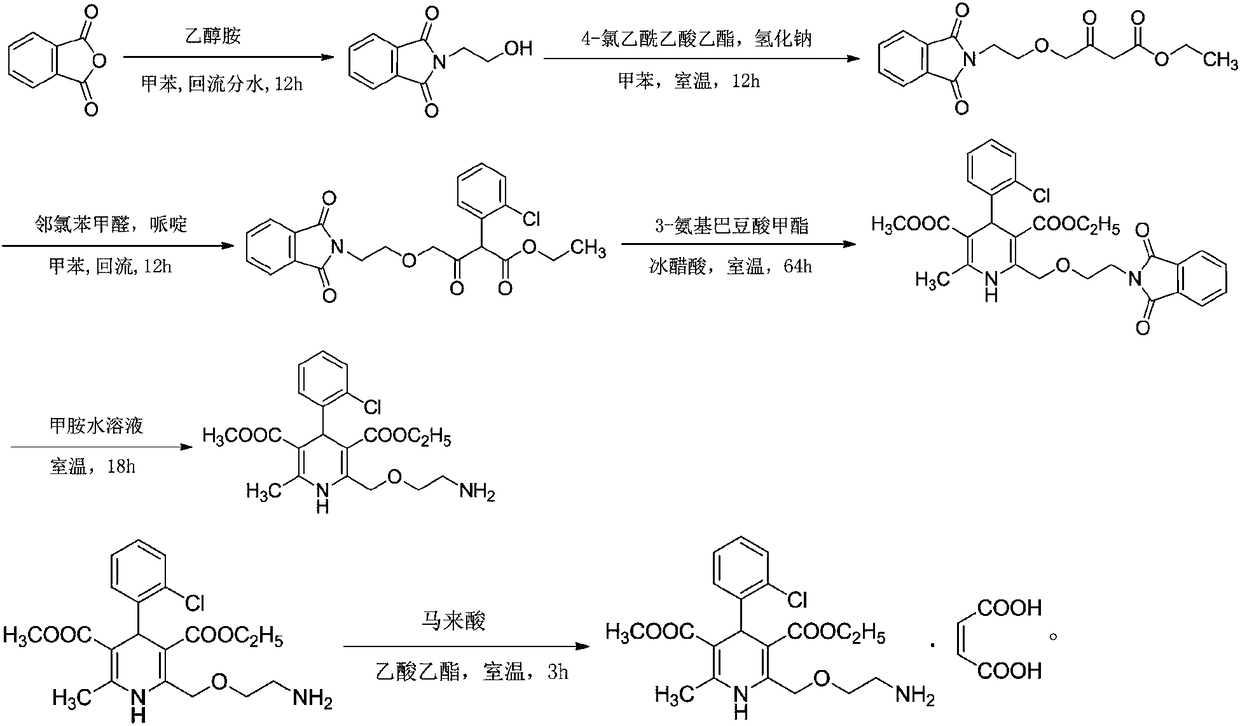
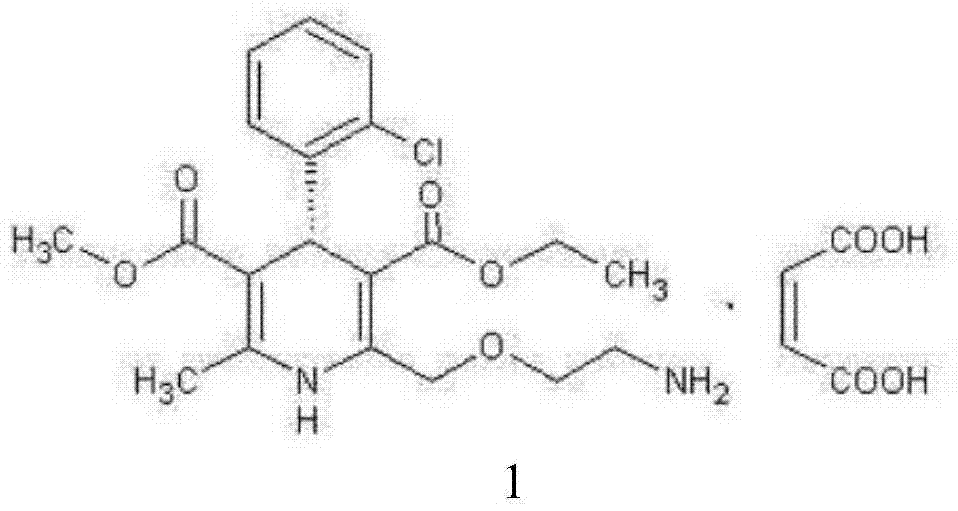
Synthesis technology of amlodipine maleate
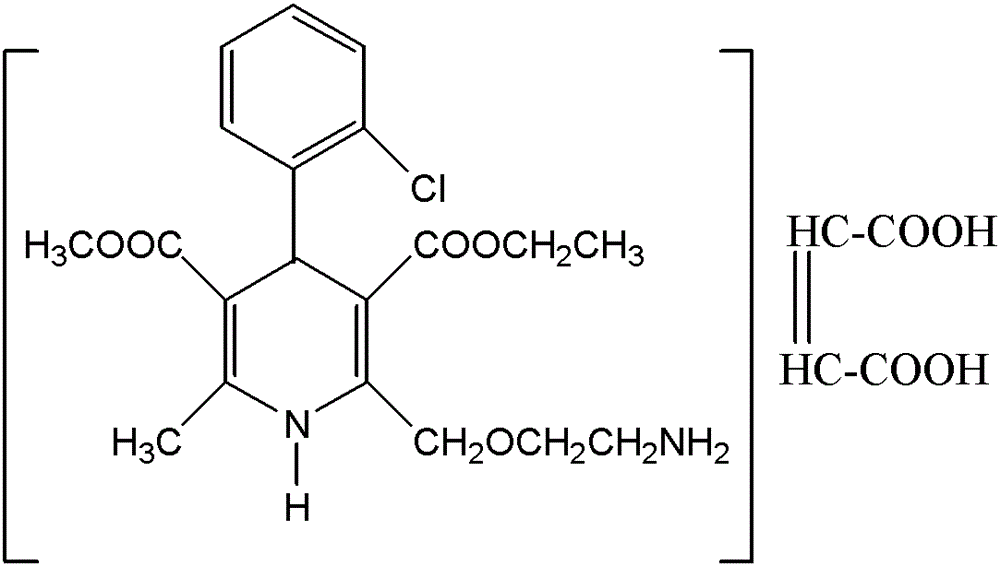
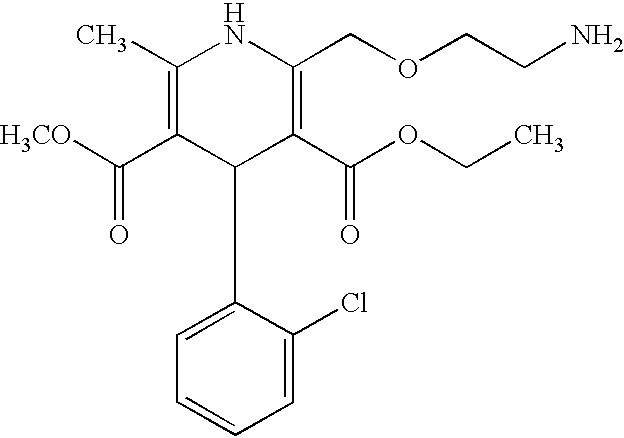
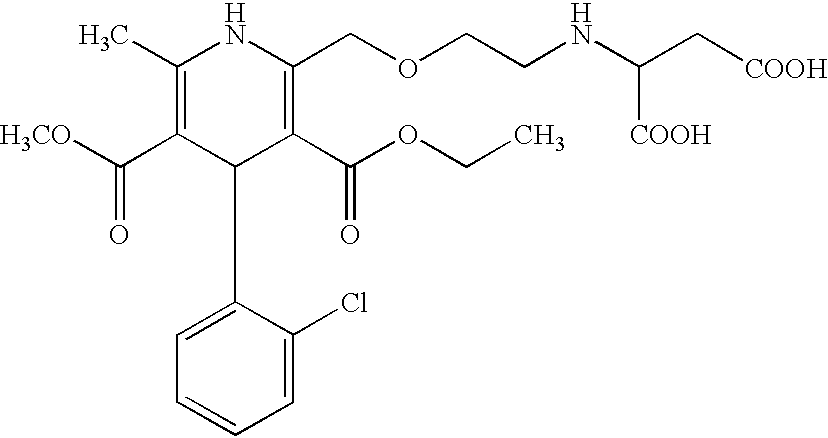
InactiveCN108358833AHigh purityLow impurity contentOrganic chemistryAmlodipine MaleateControllability
The invention discloses a synthesis technology of amlodipine maleate and relates to the technical field of medicine synthesis, aiming at solving the problem in an existing synthesis technology that more byproducts and impurities exist in industrial production. By controlling parameters of the synthesis technology and reducing the content of the impurities, the purity of the prepared amlodipine maleate reaches 99.5 percent; the self-made amlodipine maleate is used as a raw material for further preparing the amlodipine maleate, so that the cost of a product is reduced and the quality controllability of the product is strong.
Owner:上海峰林生物科技有限公司
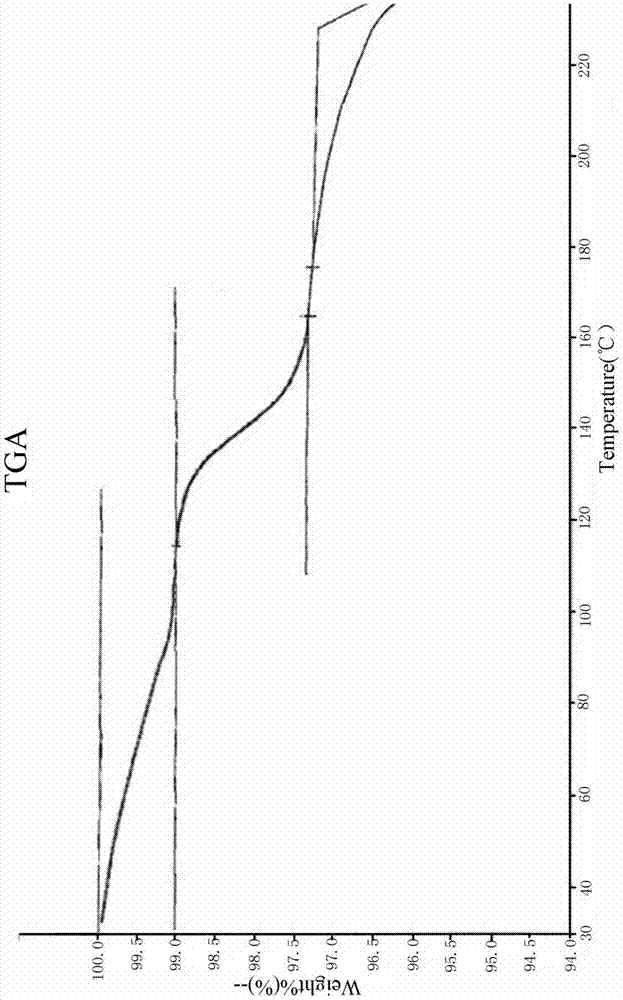
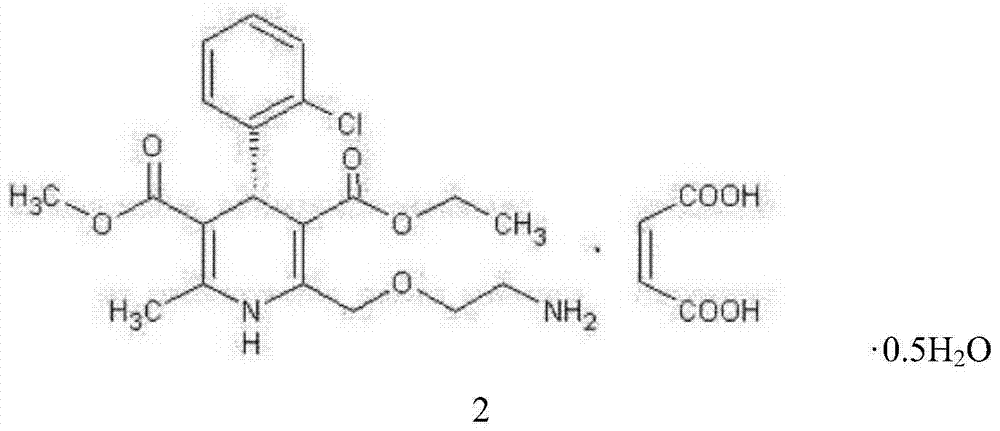
L-amlodipine maleate compound, and preparation method and medicinal preparation thereof
ActiveCN104326970AQuality improvementIncrease production capacityOrganic active ingredientsOrganic chemistryElement analysisAmlodipine Maleate
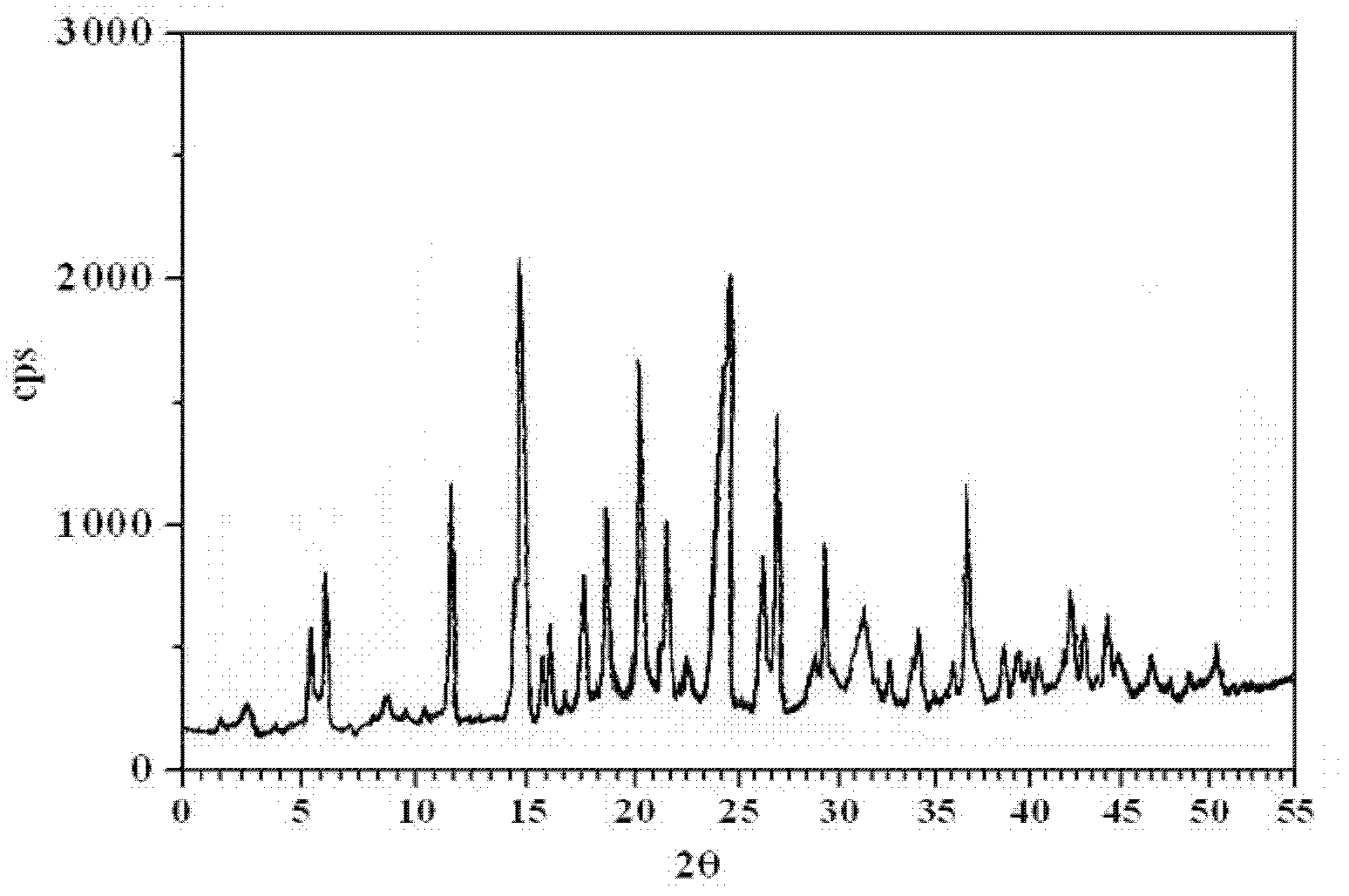
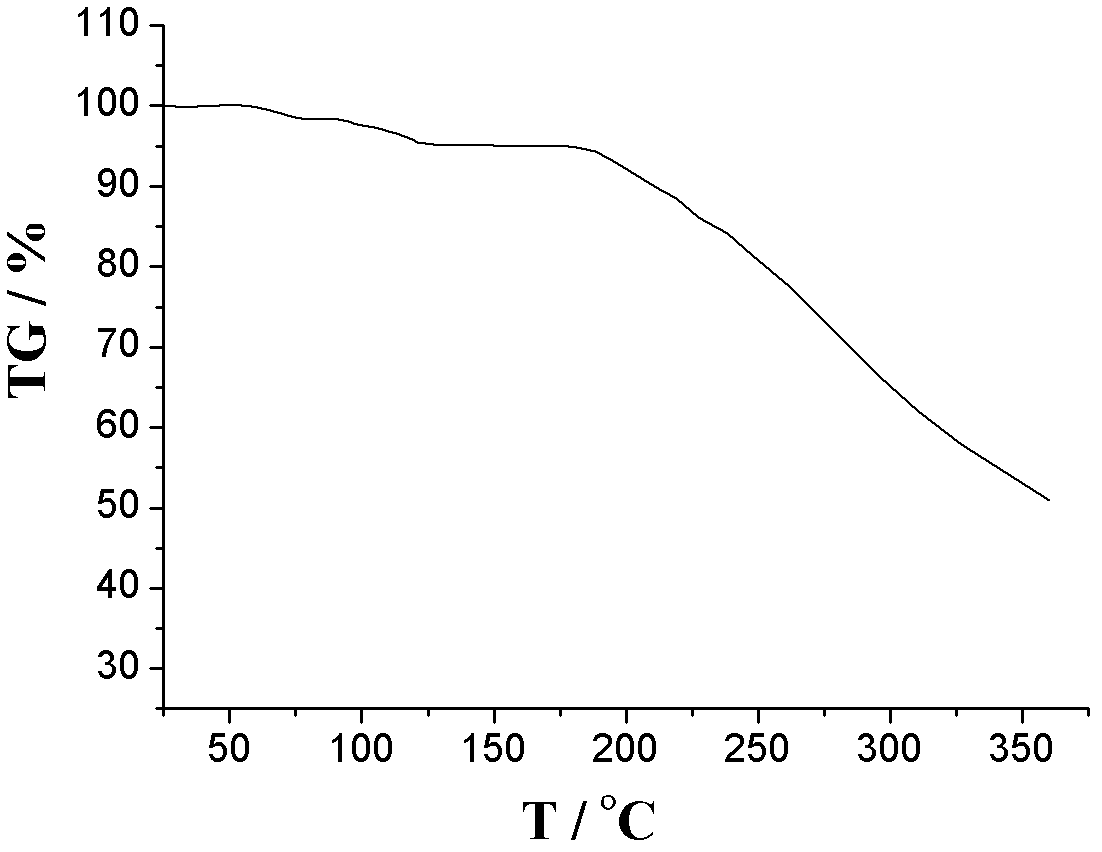
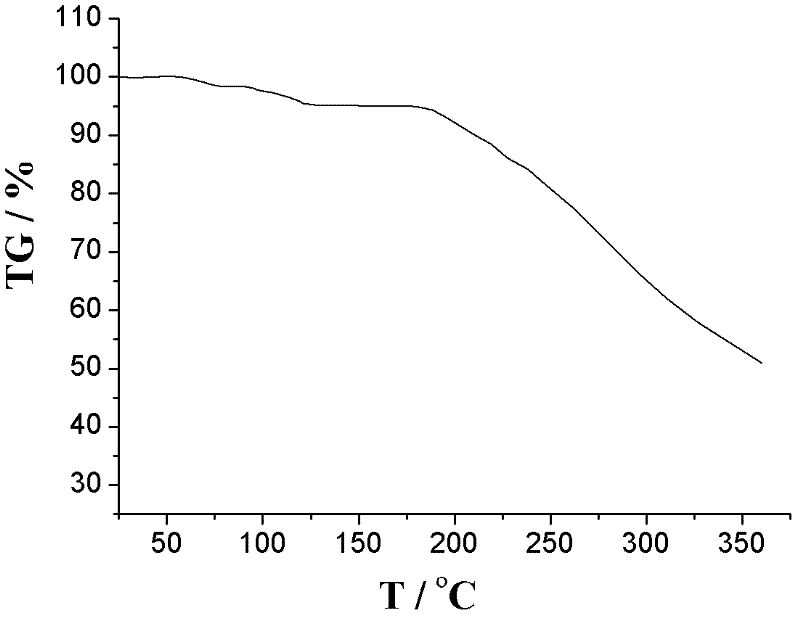
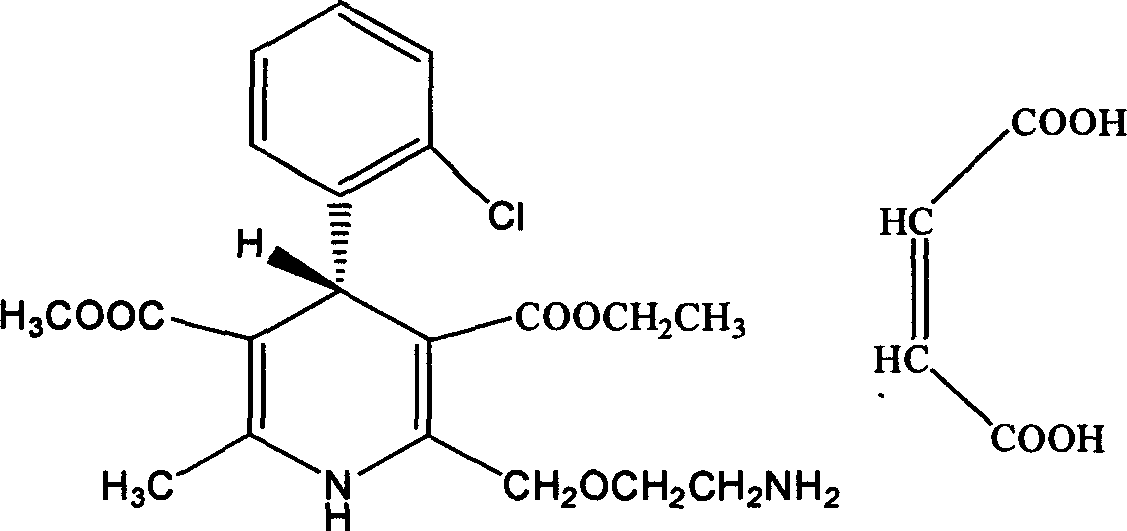
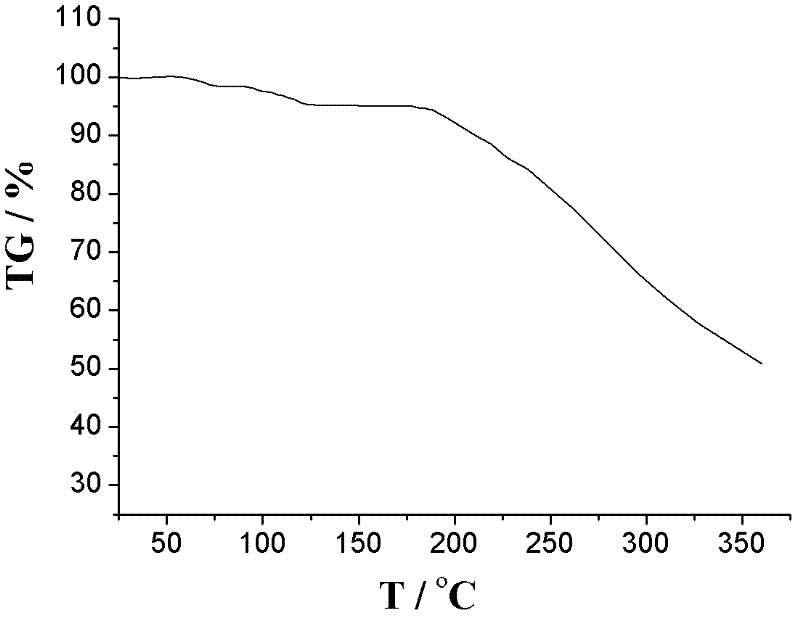
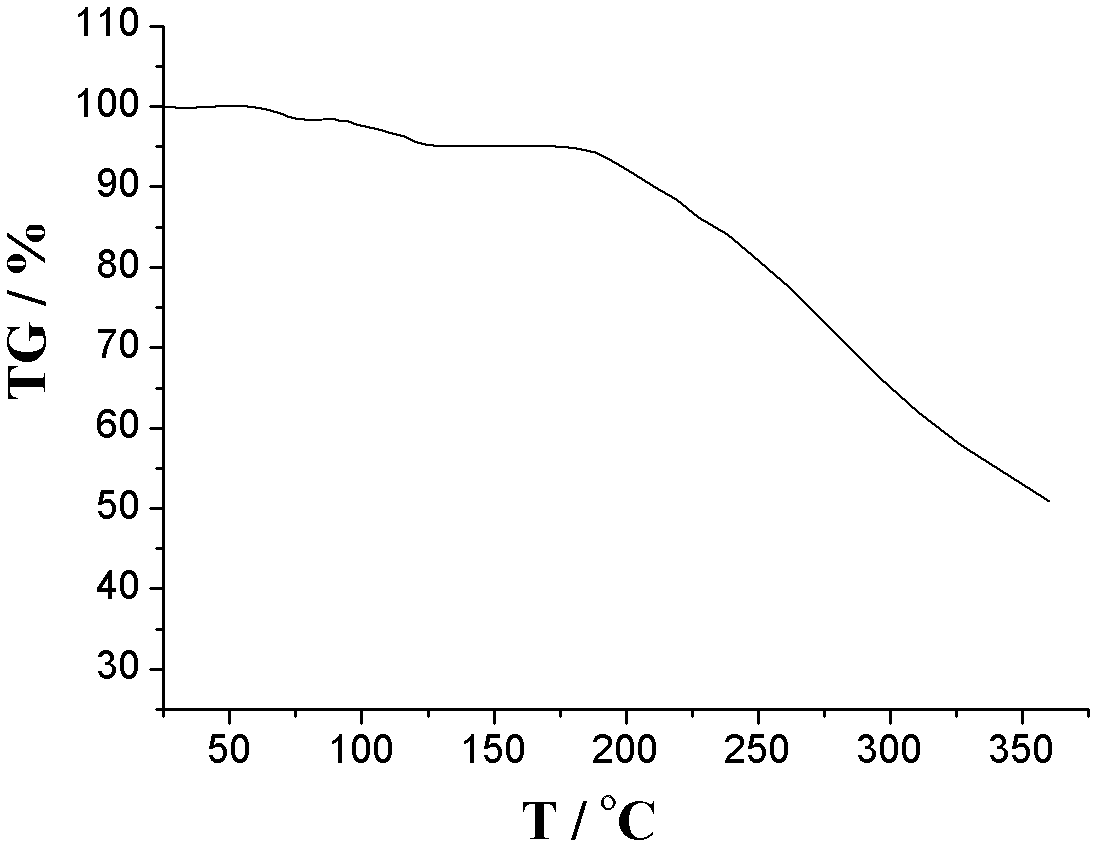
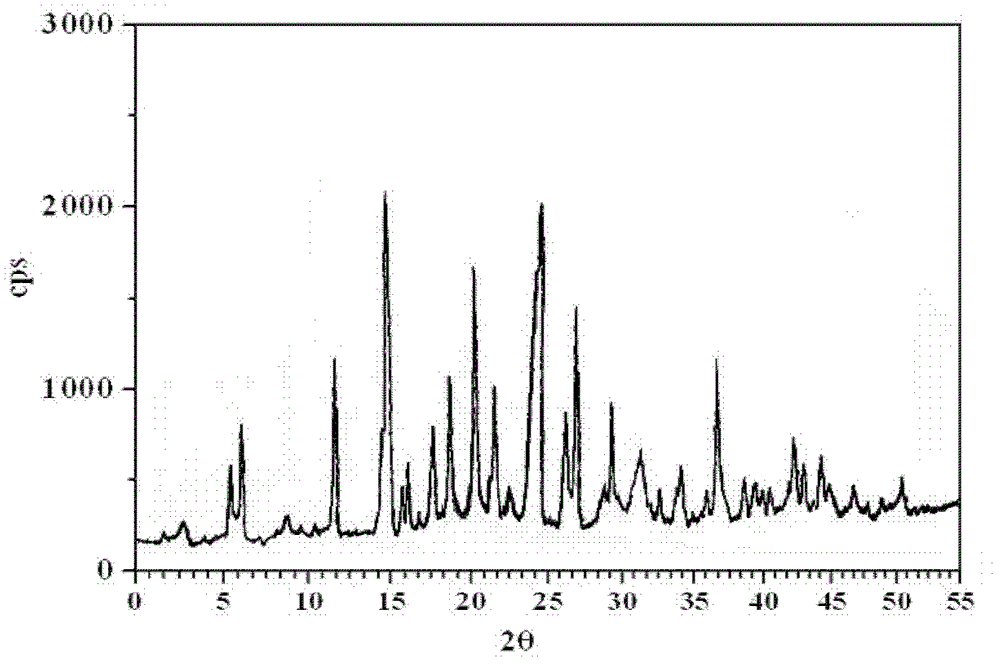
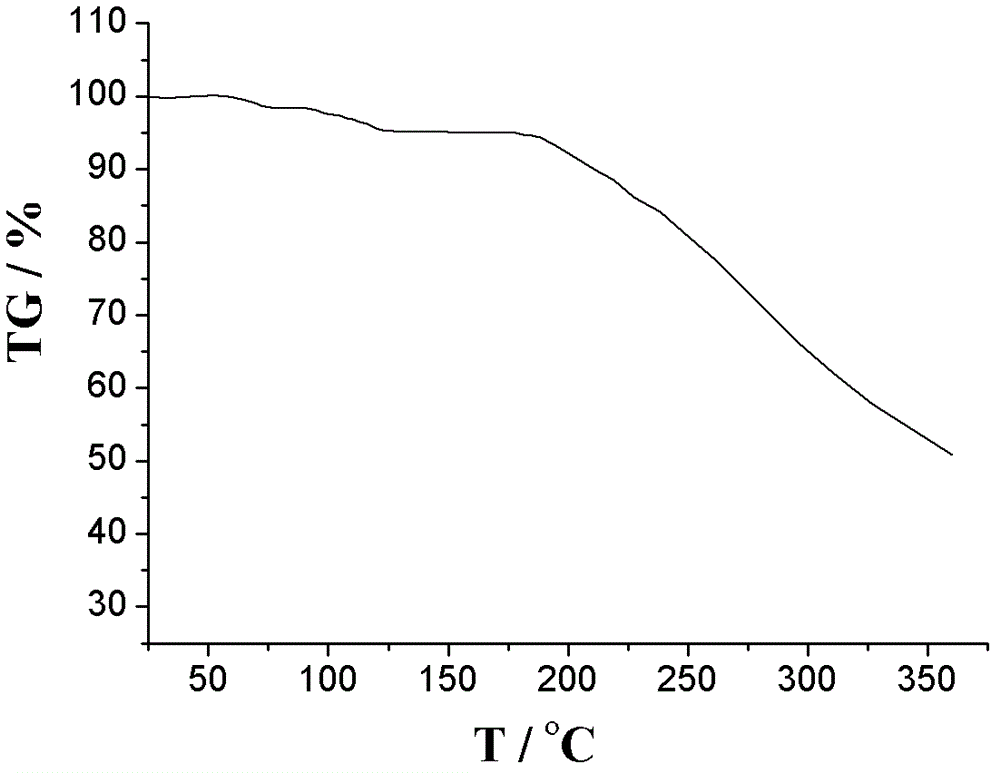
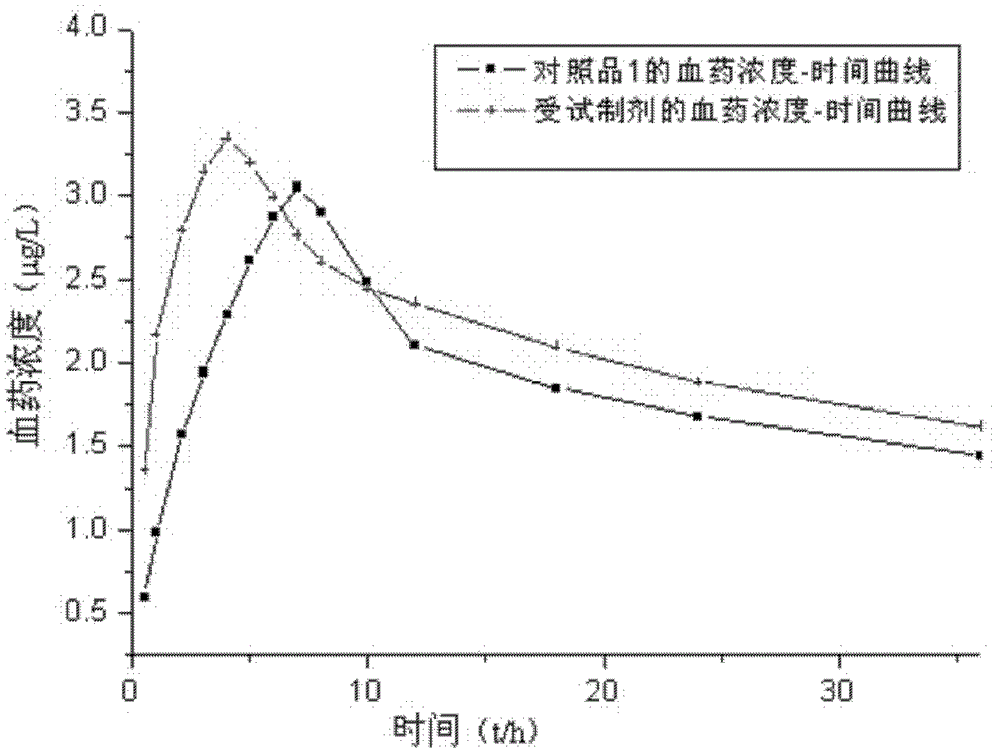
The invention relates to an L-amlodipine maleate compound, and a preparation method and a medicinal preparation thereof, and belongs to the technical field of medicines. Element analysis, water analysis and thermogravimetric analysis (TGA) detection of the L-amlodipine maleate compound prepared in the invention determines that the molecular formula of the L-amlodipine maleate compound is C20H25ClN2O5.C4H4O4.0.5H2O. Compared with products in the market, the compound has better stability to light, temperature and humidity, is more suitable for preparing various forms of medicinal preparations, such as an L-amlodipine maleate tablet and an L-amlodipine maleate dispersible tablet, as a raw medicine.
Owner:CSPC OUYI PHARM CO LTD
Amlodipine and losartan potassium medicinal composition and preparation method thereof
ActiveCN102335172ALarge specific surface areaIncrease dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsCarboxymethyl starchPotassium
The invention relates to an amlodipine and losartan potassium medicinal composition, which comprises the following components calculated according to weight part: 2.5-10 parts of amlodipine, 20-100 parts of losartan potassium, 5-20 parts of pregelatinized starch, 20-60 parts of microcrystalline cellulose, 15-40 parts of low substitution hydroxypropyl cellulose, 1-8 parts of carboxymethyl starch sodium and 1-3 parts of magnesium stearate. The amlodipine is an amlodipine maleate hydrate crystal with a molecular formula of C24H29ClN2O9.1.5H2O. The amlodipine maleate in the medicinal composition can be used for stably releasing the medicinal effect within 24 hours and stably and quickly taking effect. The medicinal composition has strong synergism, accumulation and complementary action and high bioavailability.
Owner:HAINAN JINRUI PHARMA
Maleic acid levo amido chloro diping oral disintegration tablet and its preparation method
ActiveCN1686122APromote dissolutionFully dissolvedOrganic active ingredientsPill deliveryLevamlodipineAmlodipine Maleate
An oral disintegrating tablet of levo-amlodipine maleate is prepared from levo-amlodipine maleate, water-soluble filler, disintegrant, lubricant, and optional others.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Multi-unit release pharmaceutical composition of amlodipine maleate and preparation method of pharmaceutical composition
ActiveCN106580924AReach therapeutic concentrationsActions and effects are long-lasting and long-lastingOrganic active ingredientsPharmaceutical non-active ingredientsAdjuvantDissolution
The invention belongs to the technical field of pharmaceutical preparations, and relates to a multi-unit release pharmaceutical composition of amlodipine maleate and a preparation method of the pharmaceutical composition. The pharmaceutical composition consists of the following components in percentage by weight: 10-95% of sustained-release particles containing the amlodipine maleate, 5-90% of quick-release particles containing the amlodipine maleate and 0-5% of other adjuvants. The sustained-release particles are prepared by virtue of hot-melt extrusion and the quick-release particles are prepared by virtue of dry granulation, the sustained-release particles and the quick-release particles are mixed according to a certain proportion and a lubricant is added in accordance with a formulated amount, so that the pharmaceutical composition is prepared. The pharmaceutical composition and a preparation method thereof have the advantages that the rapid dissolution of drugs can be promoted to achieve a treatment concentration and the drugs can take lasting effects, the bioavailability of the amlodipine maleate is high, the stability of the finished product (the composition) is good, and the preparation process is simple and easy to operate, low in energy consumption, free from residue of organic solvents and easy for implementation of large-scale continuous production.
Owner:NORTHEAST PHARMA GRP
Medicinal composition of amlodipine and atorvastatin calcium and its preparation method
ActiveCN102342936ALarge specific surface areaIncrease dissolution rateMetabolism disorderDrageesActive componentAmlodipine Maleate
The invention relates to a medicinal composition by taking amlodipine and atorvastatin calcium as active components, the medicinal composition comprises amlodipine, atorvastatin calcium, a filler, a disintegrant and a lubricant; the active components are composed of 2-20 parts of amlodipine and 5-120 parts of atorvastatin calcium; the amlodipine is a maleic acid amlodipine hydrate crystal. The molecular formula of the maleic acid amlodipine hydrate crystal is C24H29C1N2O9.1.5H2O. The amlodipine in the medicinal composition has the advantages of fast effectiveness and stability, and is capable of steadily releasing the drug effect in 24 hours, the medicinal composition has strong synergy, accumulation and complementary effects, and the bioavailability is high.
Owner:HAINAN JINRUI PHARMA
Pharmaceutical composition of amlodipine and telmisartan and preparation method of pharmaceutical composition
ActiveCN102327264AStable and lasting effectImprove bioavailabilityOrganic active ingredientsCardiovascular disorderAmlodipine MaleateBioavailability
The invention relates to a pharmaceutical composition of amlodipine and telmisartan; the pharmaceutical composition comprises amlodipine, telmisartan and a pharmaceutically acceptable carrier; the pharmaceutical composition contains 2.5-10 parts by weight of amlodipine and 20-80 parts by weight of telmisartan; the amlodipine is an amlodipine maleate hydrate crystal. The molecular formula of the amlodipine maleate hydrate crystal is C24H29C1N2O91.5H2O. The amlodipine in the pharmaceutical composition acts quickly and stably, and the pharmaceutical effect can be stably released within 24 h; andthe pharmaceutical composition has strong cooperation, accumulation and complementation, and high bioavailability.
Owner:HAINAN JINRUI PHARMA
Preparation method of amlodipine besylate tablets
ActiveCN103356493BAppropriate disintegration timeImprove liquidityOrganic active ingredientsPharmaceutical non-active ingredientsAmlodipine MaleateAmlodipine besilate
The invention provides a preparation method of amlodipine besylate tablets. The preparation method comprises the following steps of: employing wet granulation and tabletting, filtering each component in a formula through an 80-mesh sieve, and then drying the filtered components at 50 DEG C for future use; evenly mixing amlodipine besylate, microcrystalline cellulose and crospovidone together, forming a soft material from the mixture by using 5% starch slurry, granulating through an 18-mesh sieve, drying at 50 DEG C, and then shaping the granules through the 18-mesh sieve, thereby obtaining granules I; next, evenly mixing starch with dextrin, forming a soft material from the mixture by using 5% starch slurry, granulating through the 18-mesh sieve and drying at 50 DEG C, and then shaping the granules through the 18-mesh sieve, thereby obtaining granules II; finally, mixing the granules I with the granules II, adding magnesium stearate and evenly mixing all the materials, and then tableting the mixture, thus obtaining the amlodipine besylate tablets. According to the invention, the major component amlodipine besylate tablets are white crystalline powder which is good in fluidity; the prepared granules are excellent in both compressibility and fluidity. The disintegration time of the tablets is appropriate, and the content of the tablets, the content uniformity and the dissolution rate all meet the requirements; therefore, the tablets have high clinical application value. The method provided by the invention is simple and suitable for industrial production.
Owner:SHANGHAI SINE PROMOD PHARMA
Amlodipine besylate sustained-release capsules
InactiveCN101468004AStable blood concentrationProlong the action timeOrganic active ingredientsPharmaceutical delivery mechanismSide effectSustained Release Capsule
The invention belongs to the novel technical field of medicament and relates to a novel dosage form of amlodipine besylate sustained release capsules used for treating high blood pressure, chronic stable angina and variant angina and composed of amlodipine besylate, sugar spheres, sustained release agent, plasticizer and antisticking agent between which the weight ratio is 10:100:(0-20):(0-20):(0-25). Ethanol is used to prepare slurry, and weight of the antisticking agent is 1% that of the ethanol. The preparation technique comprises steps of preparing medicament containing spheres by granule coater, after drying, coating sustained release coating layer, drying by blowing, packing in hard capsules, thus obtaining the Manidipine sustained release capsules. Each capsule contains Manidipine 10mg-100mg, preferably 20mg-60mg. The inventive Chinese traditional medicine is slowly released so as to maintain relatively stable blood concentration and longer action time, and has advantages of reducing side effect, more convenient administration etc.
Owner:BEIJING HOPE HUGE PHARM SCI
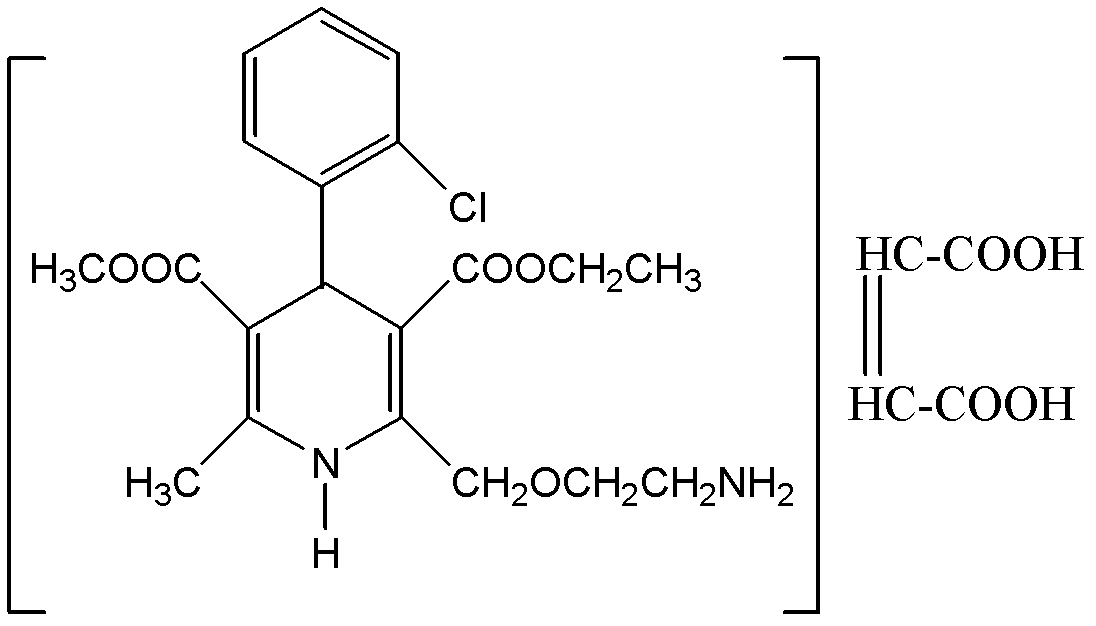
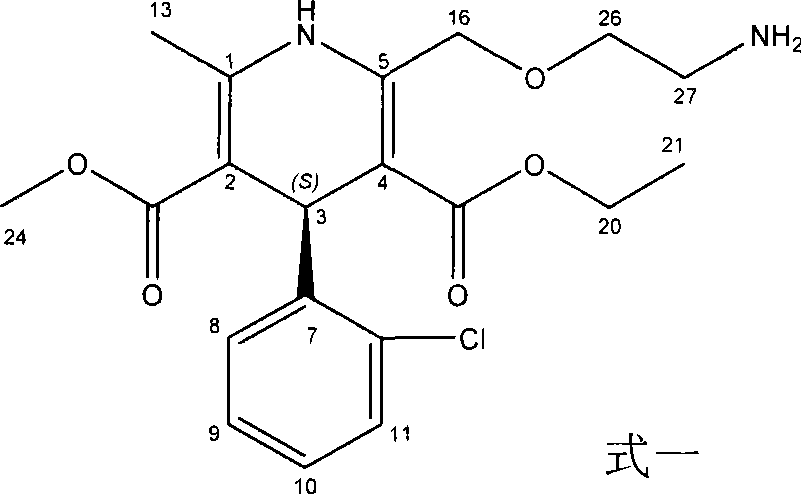
A kind of preparation method of amlodipine maleate
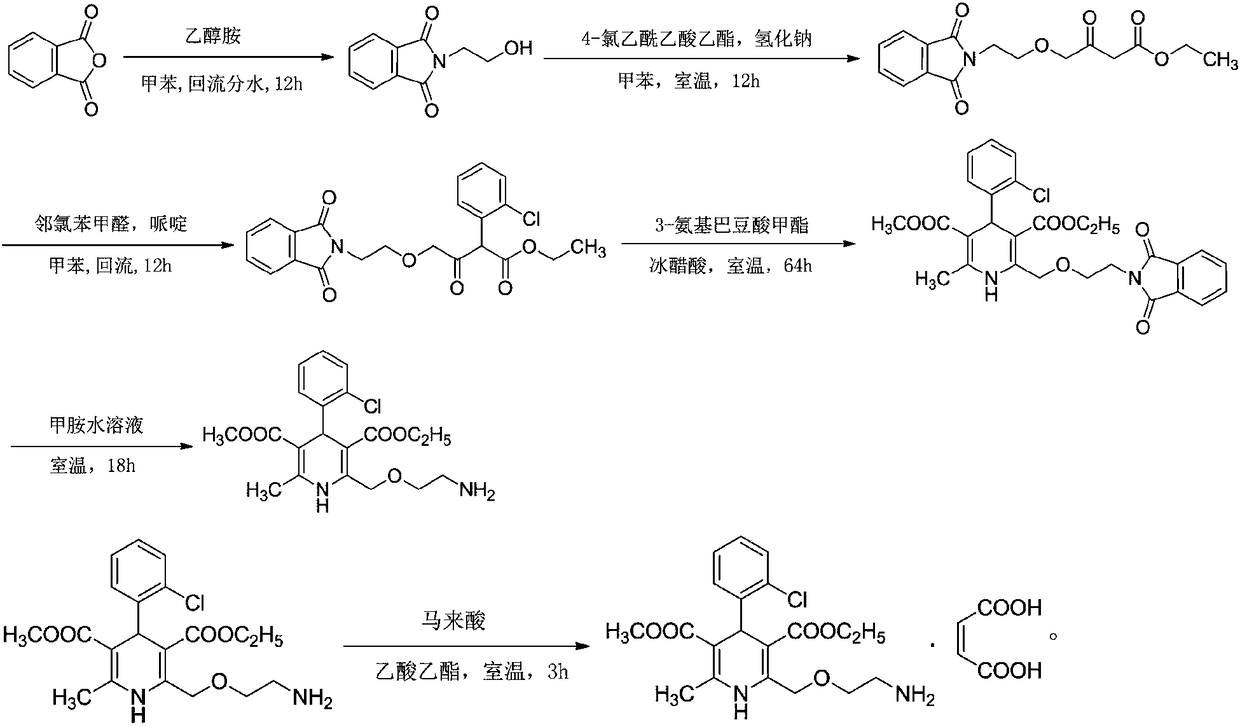
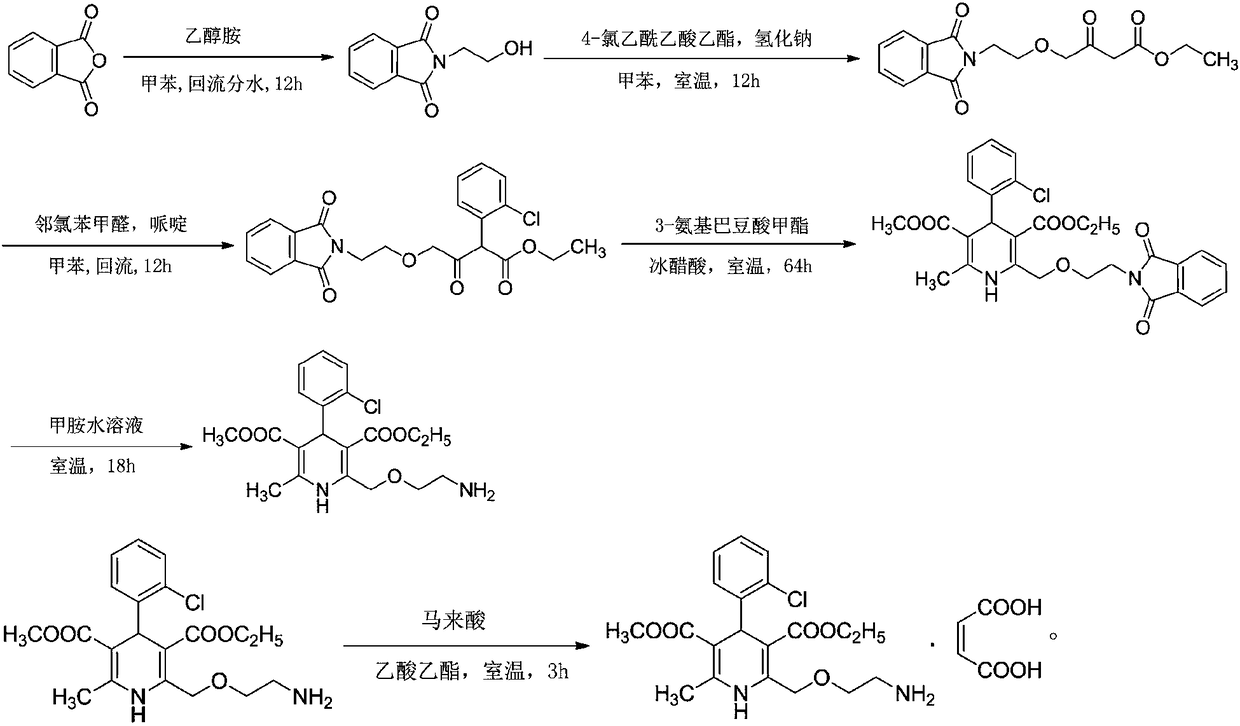
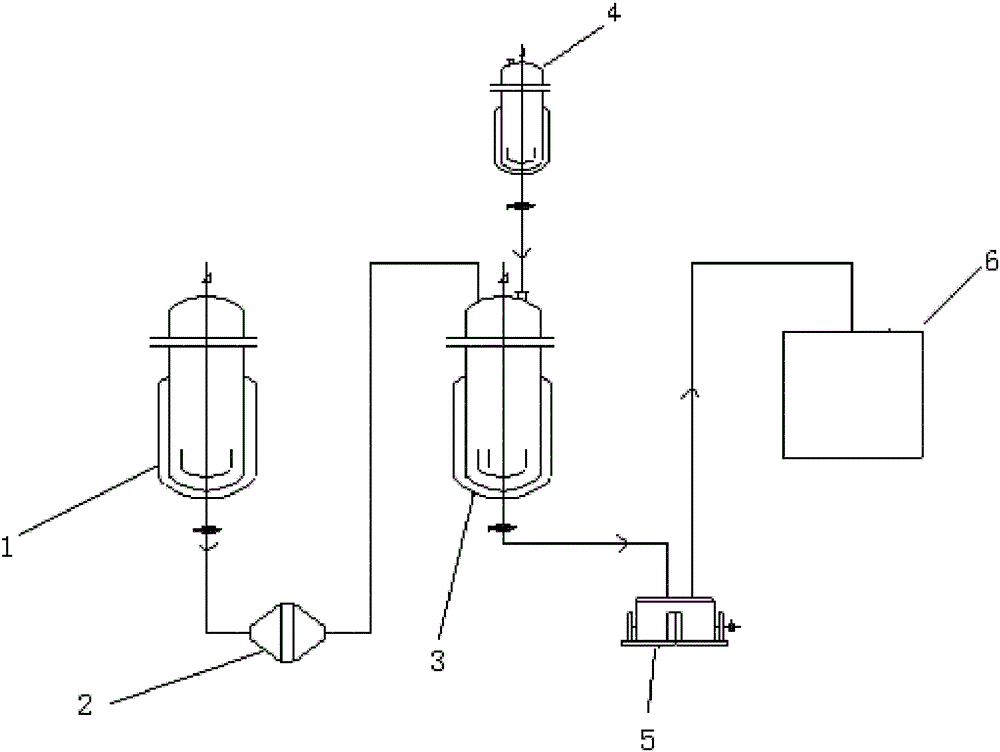
The invention relates to a preparation method of amlodipine maleate. The maleic acid and amlodipine free base are used as starting materials, the maleic acid is added to anhydrous methanol or mother liquor, heated to dissolve, and filtered to obtain a maleic acid methanol solution Add amlodipine free base to anhydrous methanol to dissolve, stir until it is completely dissolved and transparent to obtain amlodipine free base methanol solution; slowly dropwise add the obtained amlodipine free base methanol solution to the obtained maleic acid methanol solution Incubate stirring, cooling, stand for crystallization; the obtained crystals are washed with absolute ethanol and dried to obtain the finished product. The invention establishes an anhydrous methanol reaction system for amlodipine maleate salt formation, the crystal form of the finished product is greatly improved, the vacuum filtration is easy, and the yield is improved, and the mother liquor obtained by filtration after crystallization is used as the next batch of amlodipine to dissolve The solid solvent reduces the consumption of anhydrous methanol, improves the utilization rate of the solvent, saves the cost of solvent treatment, shortens the drying time, and greatly shortens the operation time.
Owner:NORTHEAST PHARMA GRP
Amlodipine fumarate
Amlodipine fumarate salt compounds are useful as calcium channel blockers and in treating or preventing angina or hypertension. The fumarate salts avoid the formation of certain potential impurities that have been found to be associated with amlodipine maleate.
Owner:SYNTHON IP
Preparation method of valsartan and amlodipine tablets and valsartan and amlodipine tablets
ActiveCN109010338AIncrease surface areaImprove adhesionPharmaceutical non-active ingredientsCoatingsValsartanMedicine
The invention discloses a preparation method of valsartan and amlodipine tablets and the valsartan and amlodipine tablets. The preparation method comprises the following steps: crushing valsartan andamlodipine benzenesulfonate for 0.5 to 5min at a rotary speed of 10000 to 30000rpm to obtain premix; mixing the premix with other auxiliary materials to obtain a primary mixed material; carrying out dry-process granulation, total mixing, tabletting and coating on the primary mixed material to prepare the valsartan and amlodipine tablets. The surface area of the valsartan is enlarged through a high-speed crushing technology, the cohesiveness is enhanced and the collapsibility is reduced; the valsartan is used for covering amlodipine and preventing the amlodipine from being dissolved out; the dissolving of the amlodipine is similar with that of an RLD, and the technical problems that the dissolving of the valsartan and amlodipine tablets prepared from a new batch of valsartan raw materials in the same factory has remarkable difference so that the dissolving of the valsartan and amlodipine tablets is not similar with that of an originally-researched valsartan and amlodipine tablet reference preparation (RLD) are solved; a preparation technology disclosed by the invention has the characteristics that the technology is scientific and reasonable, the operation is simple and easy, the production cost is relatively low and the like.
Owner:HEFEI COSOURCE PHARMA CO LTD +1
Pharmaceutical composition of amlodipine and telmisartan and preparation method of pharmaceutical composition
ActiveCN102327264BQuality improvementGood disintegrationOrganic active ingredientsCardiovascular disorderAmlodipine MaleateBioavailability
The invention relates to a pharmaceutical composition of amlodipine and telmisartan; the pharmaceutical composition comprises amlodipine, telmisartan and a pharmaceutically acceptable carrier; the pharmaceutical composition contains 2.5-10 parts by weight of amlodipine and 20-80 parts by weight of telmisartan; the amlodipine is an amlodipine maleate hydrate crystal. The molecular formula of the amlodipine maleate hydrate crystal is C24H29C1N2O91.5H2O. The amlodipine in the pharmaceutical composition acts quickly and stably, and the pharmaceutical effect can be stably released within 24 h; andthe pharmaceutical composition has strong cooperation, accumulation and complementation, and high bioavailability.
Owner:HAINAN JINRUI PHARMA CO LTD
A kind of multi-unit release pharmaceutical composition of amlodipine maleate and preparation method thereof
ActiveCN106580924BReach therapeutic concentrationsActions and effects are long-lasting and long-lastingOrganic active ingredientsPharmaceutical non-active ingredientsOrganic solventImmediate release
Owner:NORTHEAST PHARMA GRP
Amlodipine and fosinopril sodium medical composition and preparation method thereof
ActiveCN102319252BQuality improvementGood disintegrationOrganic active ingredientsDrageesAmlodipine MaleateMagnesium stearate
The invention relates to an amlodipine and fosinopril sodium medical composition, which comprises the following components in parts by weight: 5 to 10 parts of amlodipine, 10 to 40 parts of fosinopril sodium, 5 to 50 parts of compressible starch, 10 to 60 parts of microcrystalline cellulose, 15 to 40 parts of low-substitution hydroxy propyl cellulose, 10 to 45 parts of crospolyvinylpyrrolidone, and 1 to 3 parts of magnesium stearate, wherein the amlodipine is a maleic amlodipine hydrate crystal, and the molecular formula of the maleic amlodipine hydrate crystal is C24H29ClN2O9.1.5H2O. The medical composition has stable and quick action, stable and durable effect and high bioavailability.
Owner:HAINAN JINRUI PHARMA CO LTD
Amlodipine and fosinopril sodium medical composition and preparation method thereof
ActiveCN102319252ALarge specific surface areaIncrease dissolution rateOrganic active ingredientsDrageesAmlodipine MaleateMagnesium stearate
The invention relates to an amlodipine and fosinopril sodium medical composition, which comprises the following components in parts by weight: 5 to 10 parts of amlodipine, 10 to 40 parts of fosinopril sodium, 5 to 50 parts of compressible starch, 10 to 60 parts of microcrystalline cellulose, 15 to 40 parts of low-substitution hydroxy propyl cellulose, 10 to 45 parts of crospolyvinylpyrrolidone, and 1 to 3 parts of magnesium stearate, wherein the amlodipine is a maleic amlodipine hydrate crystal, and the molecular formula of the maleic amlodipine hydrate crystal is C24H29ClN2O9.1.5H2O. The medical composition has stable and quick action, stable and durable effect and high bioavailability.
Owner:HAINAN JINRUI PHARMA
Maleic acid levo amido chloro diping oral disintegration tablet and preparation method thereof
ActiveCN100508973CPromote dissolutionFully dissolvedOrganic active ingredientsPill deliveryLevamlodipineAmlodipine Maleate
An oral disintegrating tablet of levo-amlodipine maleate is prepared from levo-amlodipine maleate, water-soluble filler, disintegrant, lubricant, and optional others.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Amlodipine and olmesartan medoxomil pharmaceutical composition and preparation method thereof
ActiveCN102327265BQuality improvementGood disintegrationOrganic active ingredientsDrageesCross-linkAMLODIPINE/OLMESARTAN
Owner:HAINAN JINRUI PHARMA CO LTD
Medicinal composition of amlodipine and atorvastatin calcium and its preparation method
ActiveCN102342936BImprove medication safetyReasonable prescriptionMetabolism disorderDrageesActive componentAmlodipine Maleate
The invention relates to a medicinal composition by taking amlodipine and atorvastatin calcium as active components, the medicinal composition comprises amlodipine, atorvastatin calcium, a filler, a disintegrant and a lubricant; the active components are composed of 2-20 parts of amlodipine and 5-120 parts of atorvastatin calcium; the amlodipine is a maleic acid amlodipine hydrate crystal. The molecular formula of the maleic acid amlodipine hydrate crystal is C24H29C1N2O9.1.5H2O. The amlodipine in the medicinal composition has the advantages of fast effectiveness and stability, and is capable of steadily releasing the drug effect in 24 hours, the medicinal composition has strong synergy, accumulation and complementary effects, and the bioavailability is high.
Owner:HAINAN JINRUI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com