Amlodipine and olmesartan medoxomil pharmaceutical composition and preparation method thereof
A technology of olmesartan medoxomil and amlodipine, which is applied in the field of medicine, can solve problems such as synergy, accumulation, limited complementary effects, low overall blood drug concentration, and slow onset of amlodipine, so as to improve bioavailability, Excellent in vitro dissolution, good disintegration performance and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
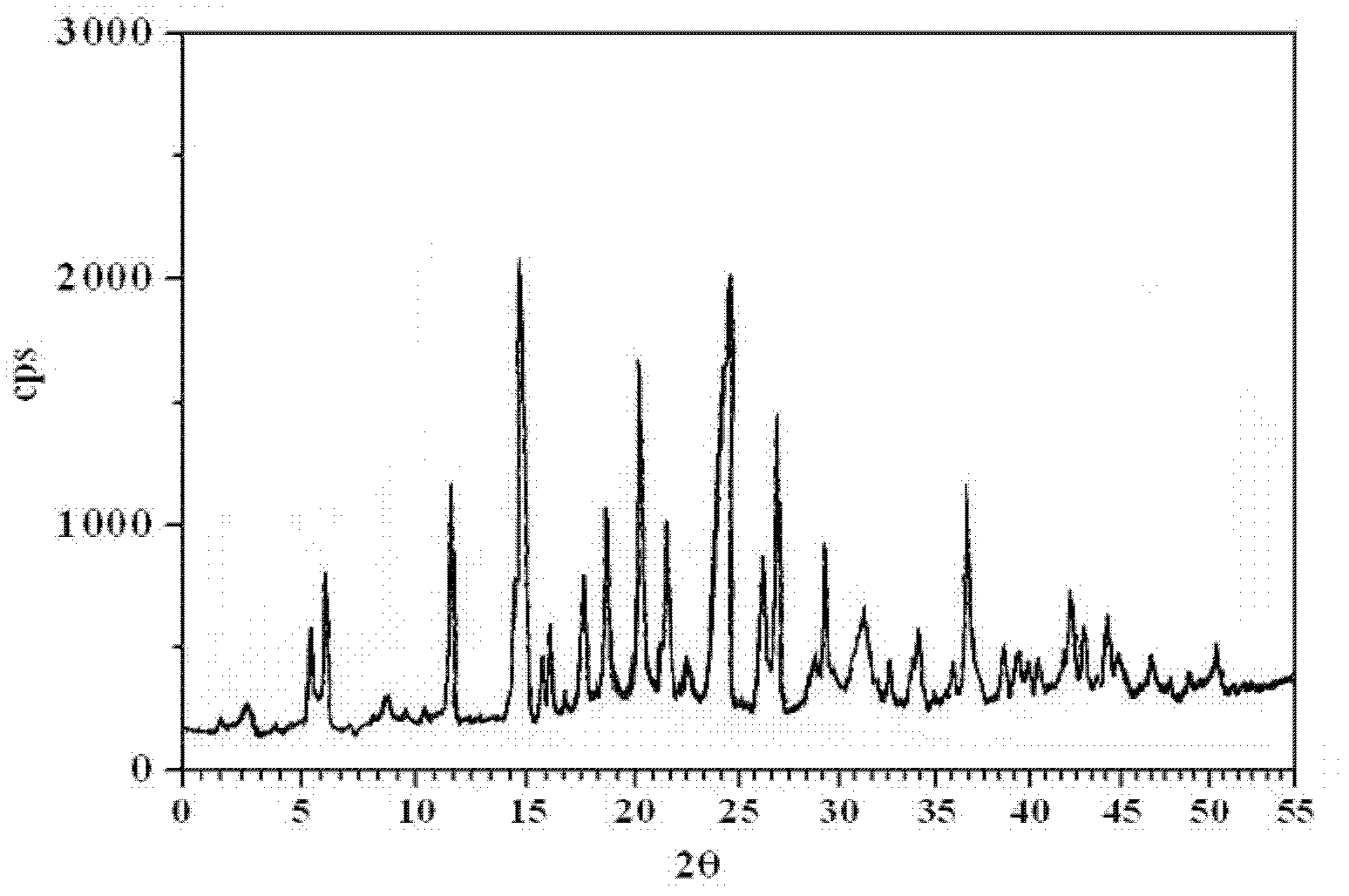
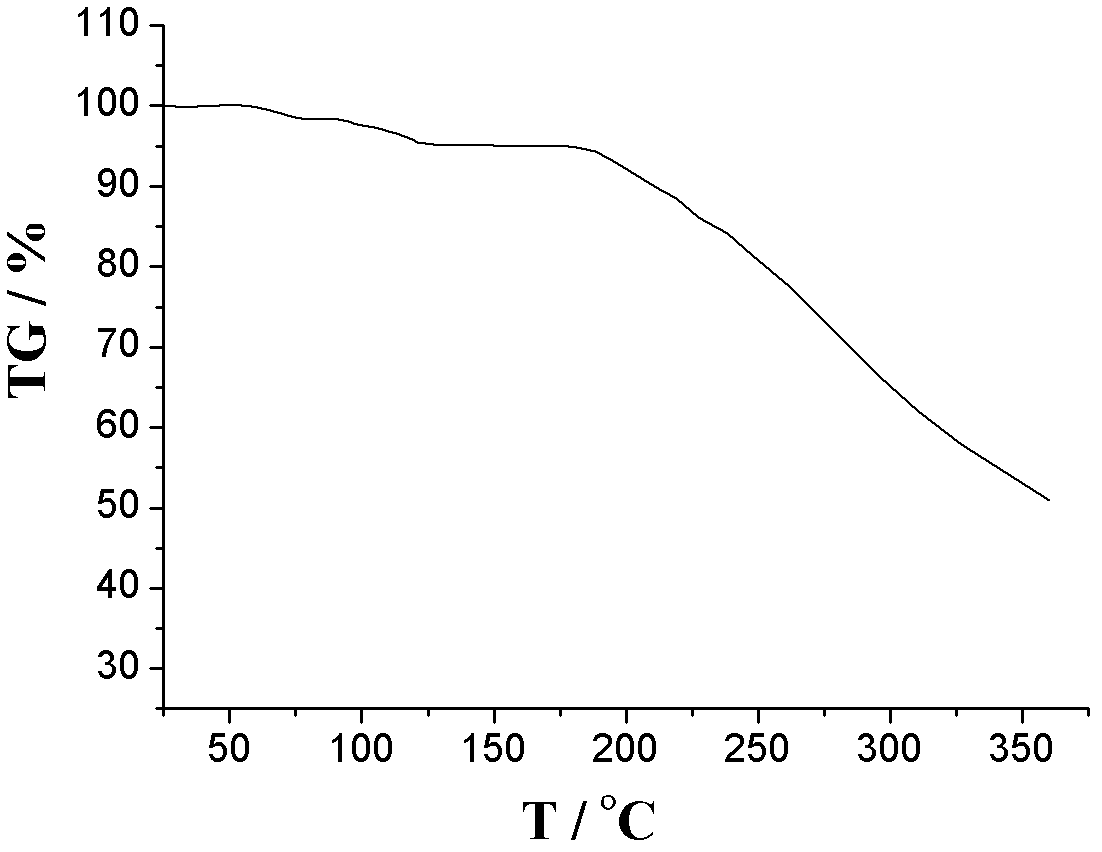
[0054] Add 9g of amlodipine maleate, 21ml of ethanol, 42ml of dimethyl sulfoxide, and 7ml of deionized water into a 100ml reaction kettle, adjust the pH to 6 with triethylamine or acetic acid, stir for 30min, seal it, and place it in an oven at 125°C After 3 days, take out the reaction kettle, place the reaction kettle in a 40KHz ultrasonic field to cool down naturally, wait for the reaction kettle to cool down slowly to 70°C, open the reaction kettle, add 70°C deionized water dropwise, white crystalline powder precipitates, and cool to room temperature And turn off the ultrasonic wave, filter, wash with dichloromethane and ethanol, and dry in vacuum for 2 hours to obtain amlodipine maleate hydrate crystals. The particle size range of the crystal is 75-150 μm, mp: 178-180°C.
[0055] Adopt U.S. Perkin-Elmer company PE 2400II elemental analyzer, elemental analysis (%) calculated value is: C (52.22), H (5.84), Cl (6.42), N (5.08), O (30.44); Elemental analysis (%) ) found: C (5...
Embodiment 2
[0059] Add 9g of amlodipine maleate, 21ml of ethanol, 42ml of dimethyl sulfoxide, and 7ml of deionized water into a 100ml reaction kettle, adjust the pH to 6.5 with triethylamine or acetic acid, stir for 30min, seal it, and place it in an oven at 130°C After 3 days, the reactor was taken out, and the reactor was placed in a 40KHz ultrasonic field to cool down naturally. After the reactor was slowly cooled to 75°C, the reactor was opened, and deionized water at 75°C was added dropwise. White crystalline powder precipitated, and cooled to room temperature. And turn off the ultrasonic wave, filter, wash with dichloromethane and ethanol, and dry in vacuum for 3 hours to obtain amlodipine maleate hydrate crystals. The particle size range of the crystal is 75-150 μm, mp: 178-180°C.
[0060] Adopt U.S. Perkin-Elmer company PE 2400II elemental analyzer, elemental analysis (%) calculated value is: C (52.22), H (5.84), Cl (6.42), N (5.08), O (30.44); Elemental analysis (%) ) found: C (...
Embodiment 3
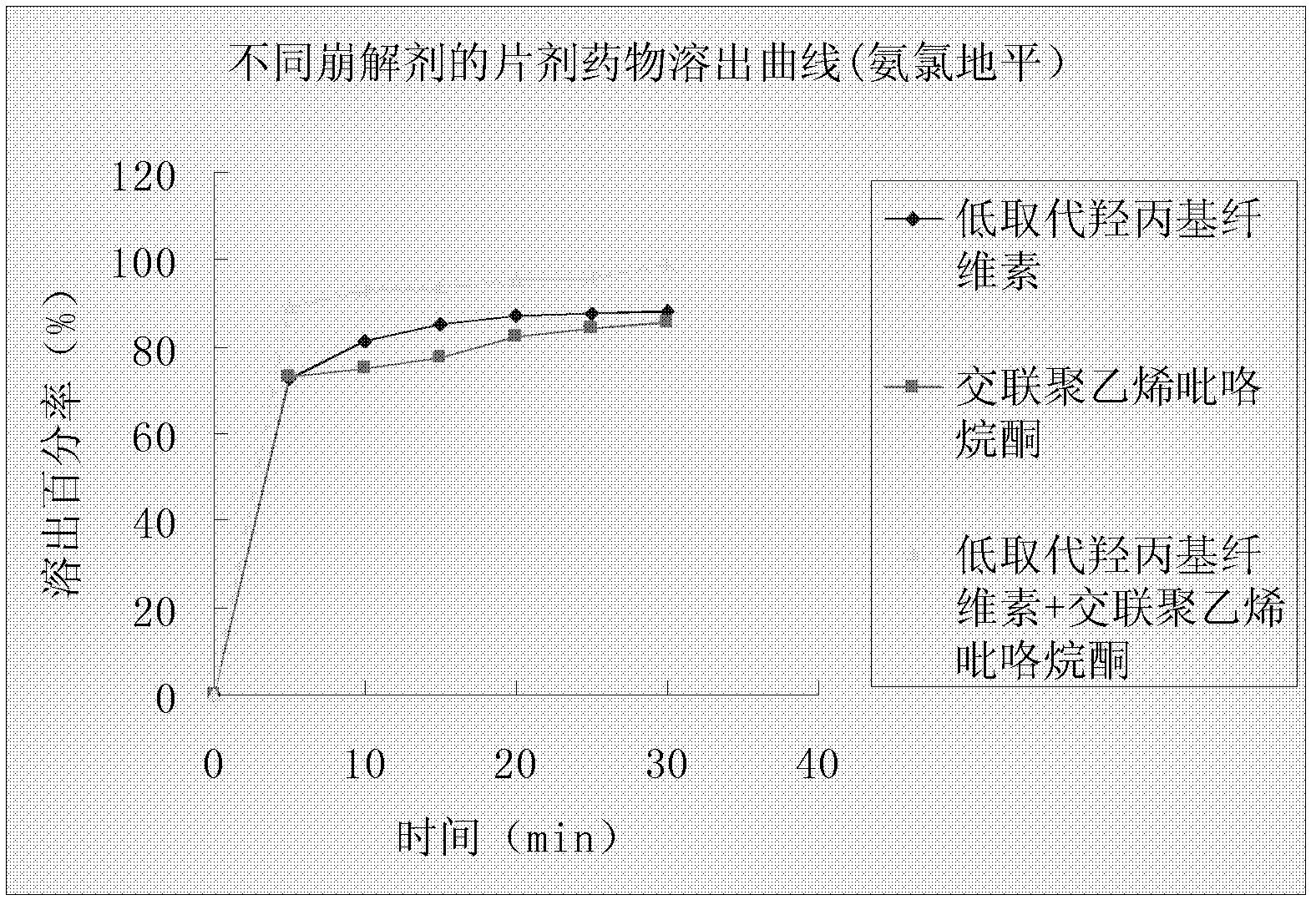
[0064] Ingredients as follows:
[0065] Material name
First group
Second Group
The third group
Fourth group
fifth group
The sixth group
seventh group
2.5
5
10g
10g
10g
10g
10g
Olmesartan medoxomil
20
30
40g
40g
40g
40g
40g
compressible starch
5
20
30g
36g
18g
50g
30g
Microcrystalline Cellulose PH102
10
15
46g
40g
60g
20g
38g
Low-substituted hydroxypropyl cellulose
15
20
32g
35g
15
28
40
Cross-linked polyvinylpyrrolidone
10
15
38g
30g
45g
30g
33g
essence
1
1.5
2g
2g
5g
2g
2g
1
1
1g
1g
3g
1g
1g
[0066] 1000 pieces a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com