Medicinal composition of amlodipine and atorvastatin calcium and its preparation method
A technology of atorvastatin calcium and amlodipine, applied in the field of medicine, can solve the problems of synergy, accumulation, limited complementary effect, synergy, accumulation, limited complementary effect, low overall level of blood drug concentration, etc., and achieves excellent dissolution performance. , the effect of improving bioavailability and good quality stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
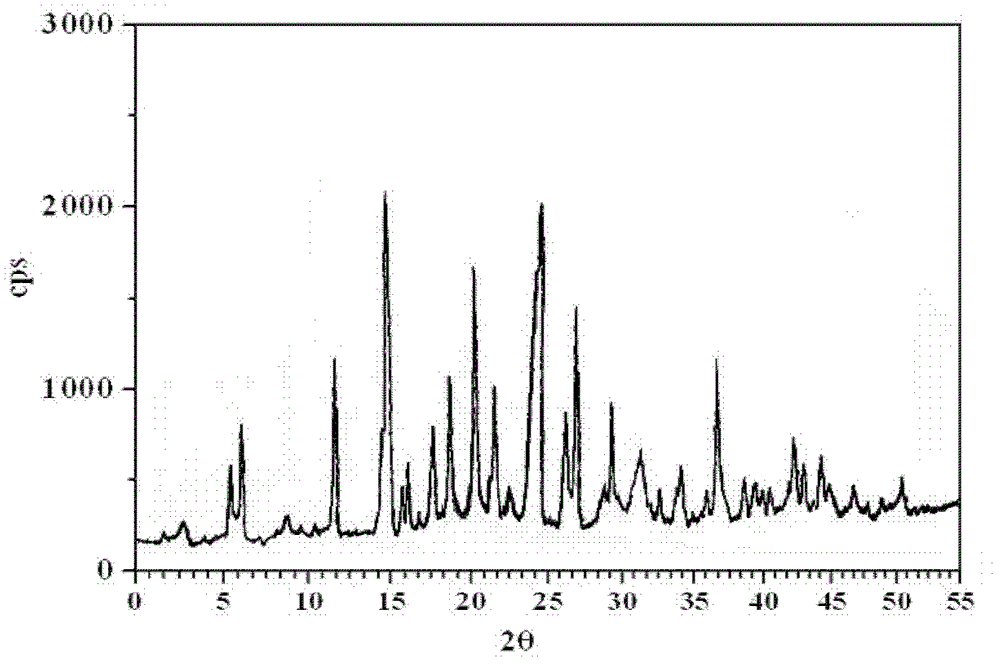
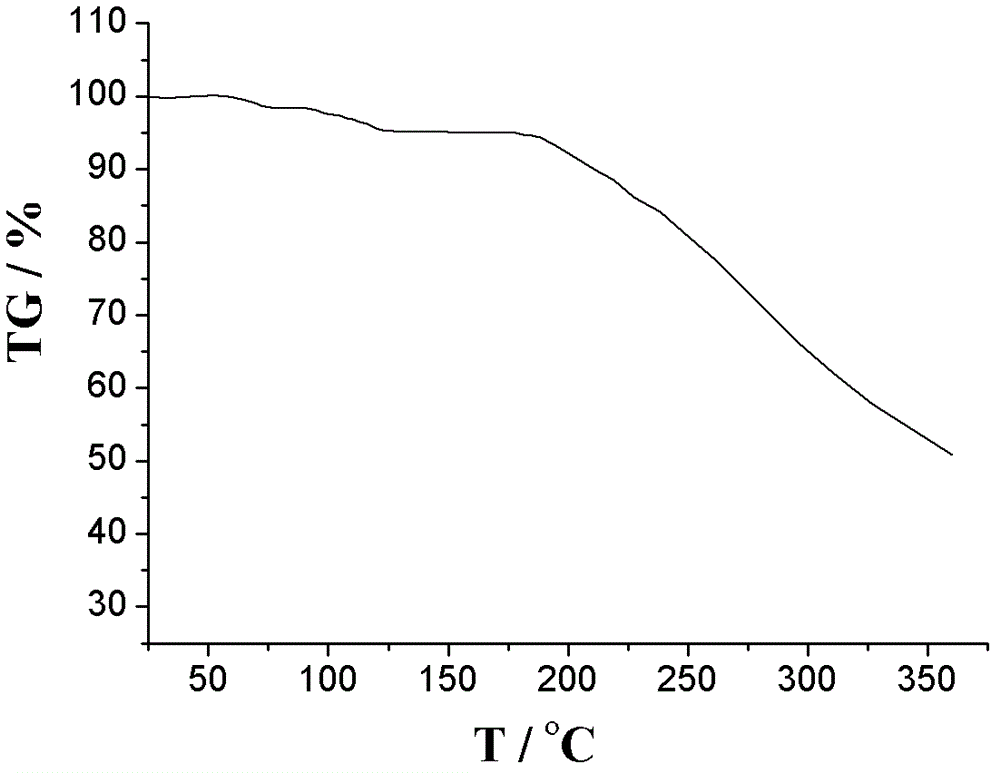
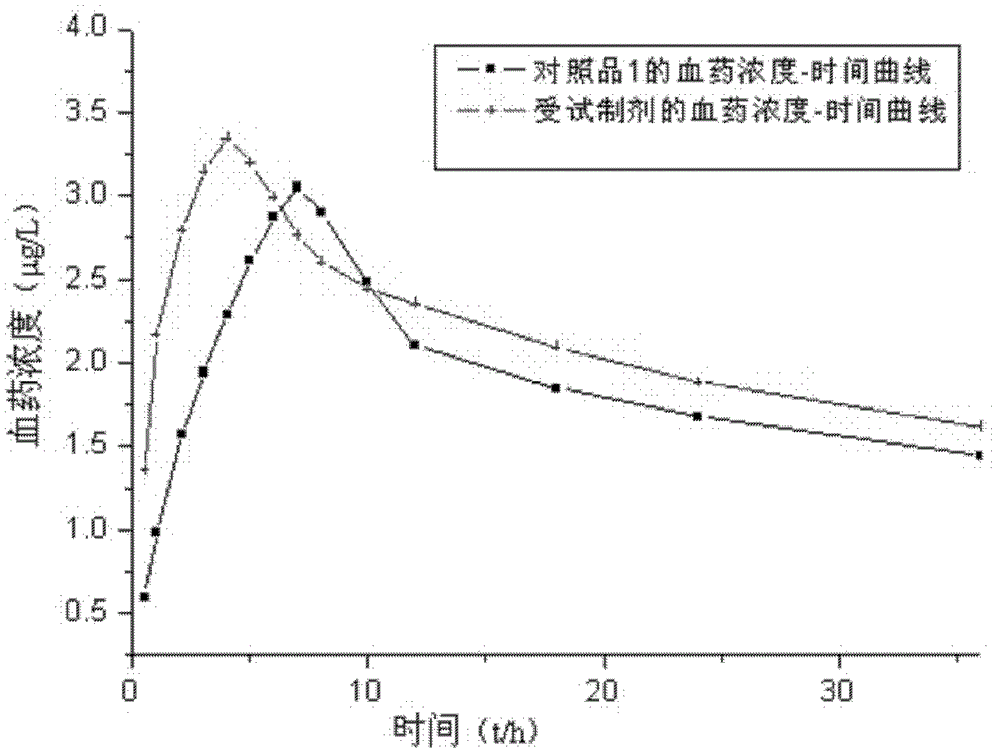
Image
Examples
Embodiment 1
[0058] Add 9g of amlodipine maleate, 21ml of ethanol, 42ml of dimethyl sulfoxide, and 7ml of deionized water into a 100ml reaction kettle, adjust the pH to 6 with triethylamine or acetic acid, stir for 30min, seal it, and place it in an oven at 125°C After 3 days, take out the reaction kettle, place the reaction kettle in a 40KHz ultrasonic field to cool down naturally, wait for the reaction kettle to cool down slowly to 70°C, open the reaction kettle, add 70°C deionized water dropwise, white crystalline powder precipitates, and cool to room temperature And turn off the ultrasonic wave, filter, wash with dichloromethane and ethanol, and dry in vacuum for 2 hours to obtain amlodipine maleate hydrate crystals. The particle size range of the crystal is 75-150 μm, mp: 178-180°C.
[0059] Adopt U.S. Perkin-Elmer company PE2400II elemental analyzer, elemental analysis (%) calculated value is: C (52.22), H (5.84), Cl (6.42), N (5.08), O (30.44); Elemental analysis (%) Found: C (52.2...
Embodiment 2
[0063] Add 9g of amlodipine maleate, 21ml of ethanol, 42ml of dimethyl sulfoxide, and 7ml of deionized water into a 100ml reaction kettle, adjust the pH to 6.5 with triethylamine or acetic acid, stir for 30min, seal it, and place it in an oven at 130°C After 3 days, the reactor was taken out, and the reactor was placed in a 40KHz ultrasonic field to cool down naturally. After the reactor was slowly cooled to 75°C, the reactor was opened, and deionized water at 75°C was added dropwise. White crystalline powder precipitated, and cooled to room temperature. And turn off the ultrasonic wave, filter, wash with dichloromethane and ethanol, and dry in vacuum for 3 hours to obtain amlodipine maleate hydrate crystals. The particle size range of the crystal is 75-150 μm, mp: 178-180°C.
[0064] Adopt U.S. Perkin-Elmer company PE2400II elemental analyzer, elemental analysis (%) calculated value is: C (52.22), H (5.84), Cl (6.42), N (5.08), O (30.44); Elemental analysis (%) Found: C (52....
Embodiment 3
[0068] Prescription: specification (amlodipine maleate hydrate crystal / atorvastatin calcium 2.5mg / 5mg)
[0069]
[0070] First pass the raw and auxiliary materials through a 60-mesh sieve, then weigh the amlodipine maleate hydrate crystals, fillers, disintegrants, and lubricants in the prescribed amount, and mix them uniformly according to the method of equal increments to obtain maleic acid Amlodipine crystalline mixture powder.
[0071] First pass the raw and auxiliary materials through a 60-mesh sieve, then weigh the prescribed amount of atorvastatin calcium, filler, and disintegrating agent, mix them evenly according to the method of equal increments, and then add the adhesive to make a soft material, 20-mesh After sieving and granulating, the wet granules were dried at 52° C., sized, and the dry granules were mixed with a lubricant to obtain atorvastatin calcium granules.
[0072] Amlodipine mixture powder and atorvastatin calcium granules are respectively placed in a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com