Amlodipine and fosinopril sodium medical composition and preparation method thereof
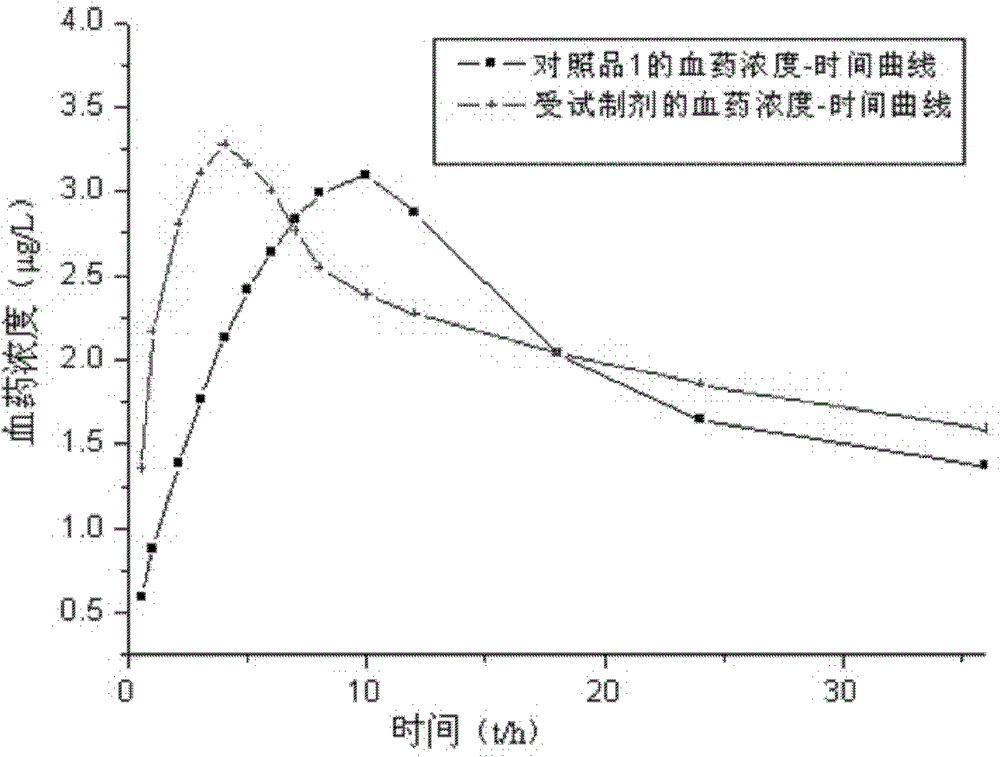
A technology of fosinopril sodium and amlodipine is applied in the field of pharmaceutical compositions of amlodipine and fosinopril sodium and their preparation, which can solve the problem of low overall level of blood drug concentration, undisclosed preparation method, increase Drug cost and other issues, to achieve the effect of improving bioavailability, good disintegration performance and dissolution, and stable and long-lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
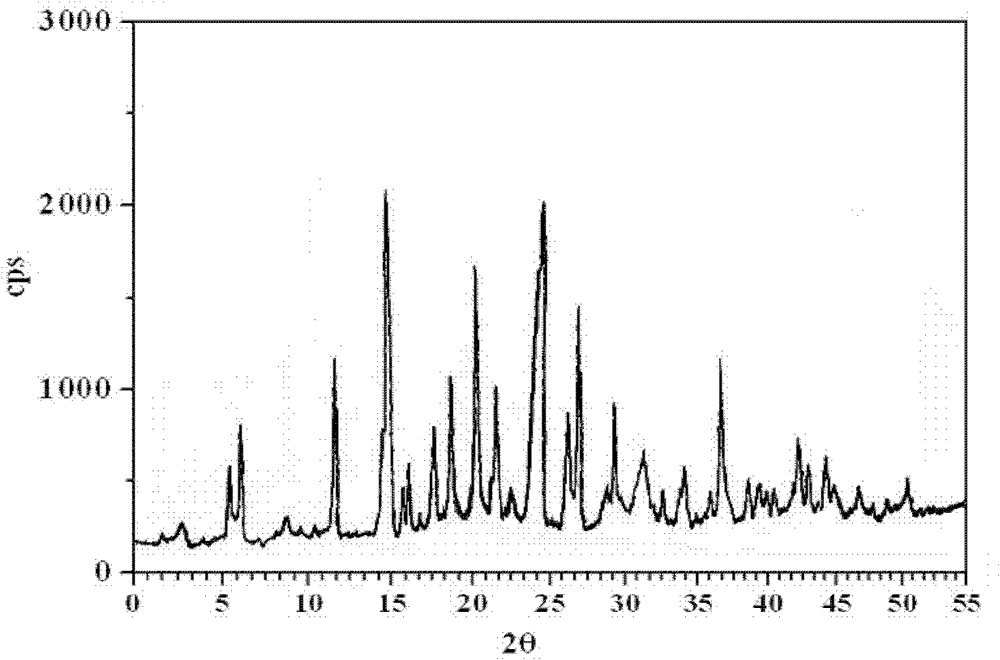
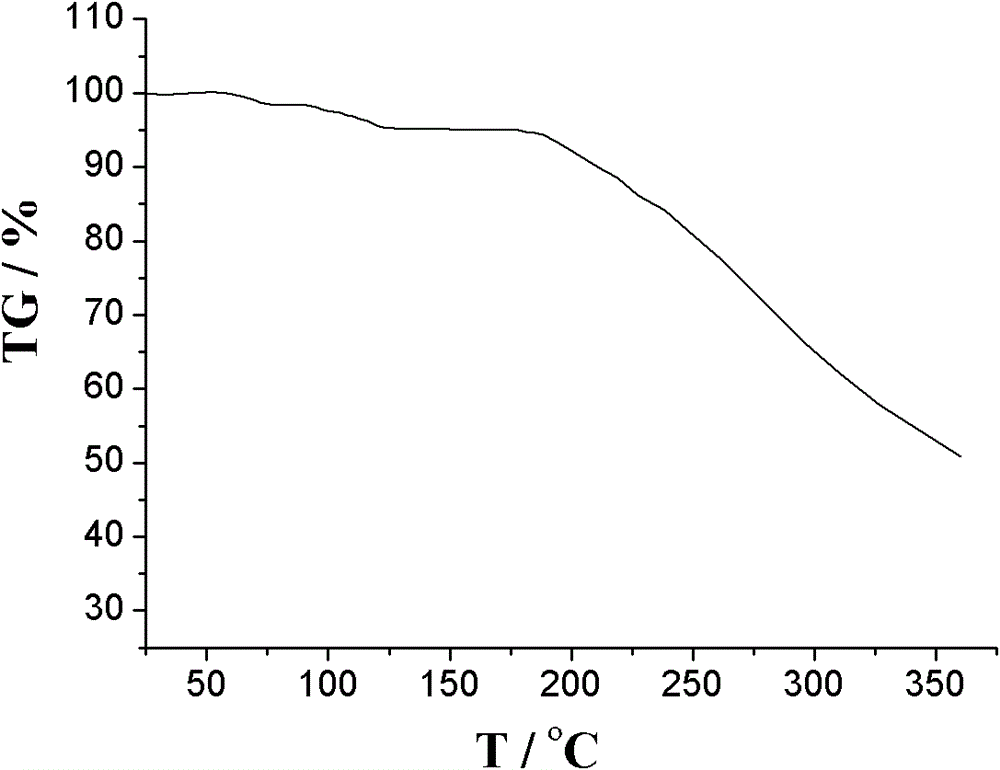
[0048] Add 9g of amlodipine maleate, 21ml of ethanol, 42ml of dimethyl sulfoxide, and 7ml of deionized water into a 100ml reaction kettle, adjust the pH to 6 with triethylamine or acetic acid, stir for 30min, seal it, and place it in an oven at 125°C After 3 days, take out the reaction kettle, place the reaction kettle in a 40KHz ultrasonic field to cool down naturally, wait for the reaction kettle to cool down slowly to 70°C, open the reaction kettle, add 70°C deionized water dropwise, white crystalline powder precipitates, and cool to room temperature And turn off the ultrasonic wave, filter, wash with dichloromethane and ethanol, and dry in vacuum for 2 hours to obtain amlodipine maleate hydrate crystals. The particle size range of the crystal is 75-150 μm, mp: 178-180°C.
[0049] Adopt U.S. Perkin-Elmer company PE 2400II elemental analyzer, elemental analysis (%) calculated value is: C (52.22), H (5.84), Cl (6.42), N (5.08), O (30.44); Elemental analysis (%) ) found: C (5...
Embodiment 2
[0053] Add 9g of amlodipine maleate, 21ml of ethanol, 42ml of dimethyl sulfoxide, and 7ml of deionized water into a 100ml reaction kettle, adjust the pH to 6.5 with triethylamine or acetic acid, stir for 30min, seal it, and place it in an oven at 130°C After 3 days, the reactor was taken out, and the reactor was placed in a 40KHz ultrasonic field to cool down naturally. After the reactor was slowly cooled to 75°C, the reactor was opened, and deionized water at 75°C was added dropwise. White crystalline powder precipitated, and cooled to room temperature. And turn off the ultrasonic wave, filter, wash with dichloromethane and ethanol, and dry in vacuum for 3 hours to obtain amlodipine maleate hydrate crystals. The particle size range of the crystal is 75-150 μm, mp: 178-180°C.
[0054] Adopt U.S. Perkin-Elmer company PE 2400II elemental analyzer, elemental analysis (%) calculated value is: C (52.22), H (5.84), Cl (6.42), N (5.08), O (30.44); Elemental analysis (%) ) found: C (...
Embodiment 3
[0058] Ingredients as follows:
[0059]
[0060] 1000 pieces are made
[0061] Preparation:
[0062] Raw and auxiliary materials were respectively crushed through 80-mesh sieve, microcrystalline cellulose and amlodipine maleate hydrate crystals were mixed, and then mixed with fosinopril sodium, compressible starch and low-substituted hydroxypropyl cellulose, cross-linked The polyvinylpyrrolidone is mixed evenly, and then mixed with magnesium stearate for 5 minutes, the intermediate is tested, and the composition chip is obtained by direct tableting.
[0063] Finally, the prescribed amount of magnesium stearate and essence are added, mixed evenly, and after the intermediate is qualified, the medicinal composition chip is obtained by direct tableting; and the film coating is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com