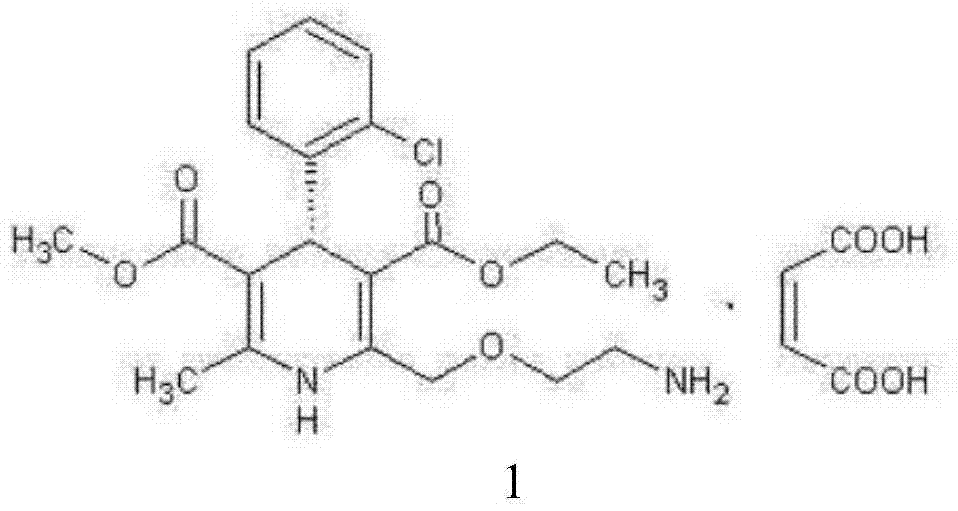
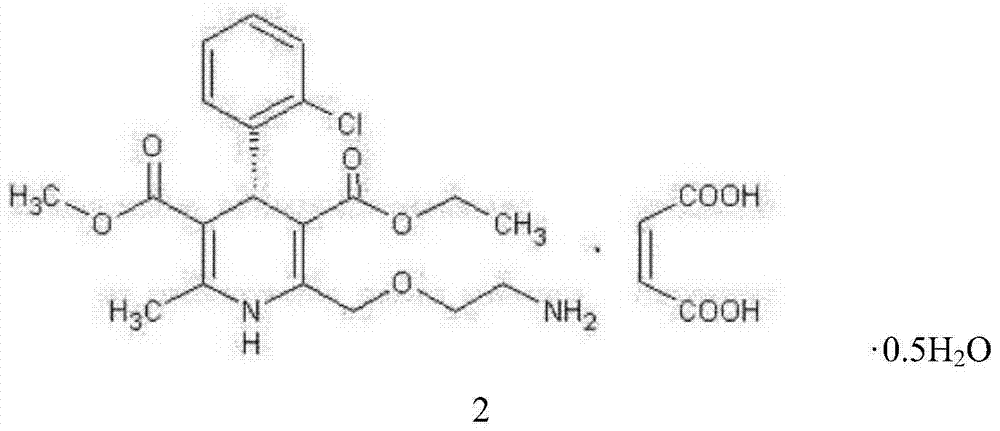
L-amlodipine maleate compound, and preparation method and medicinal preparation thereof
A technology for levamlodipine and pharmaceutical preparations, applied in the field of medicine, can solve problems such as research on the form of solid existence, inability to form hydrates, etc., and achieve the effects of being conducive to production and storage, stable quality and better quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
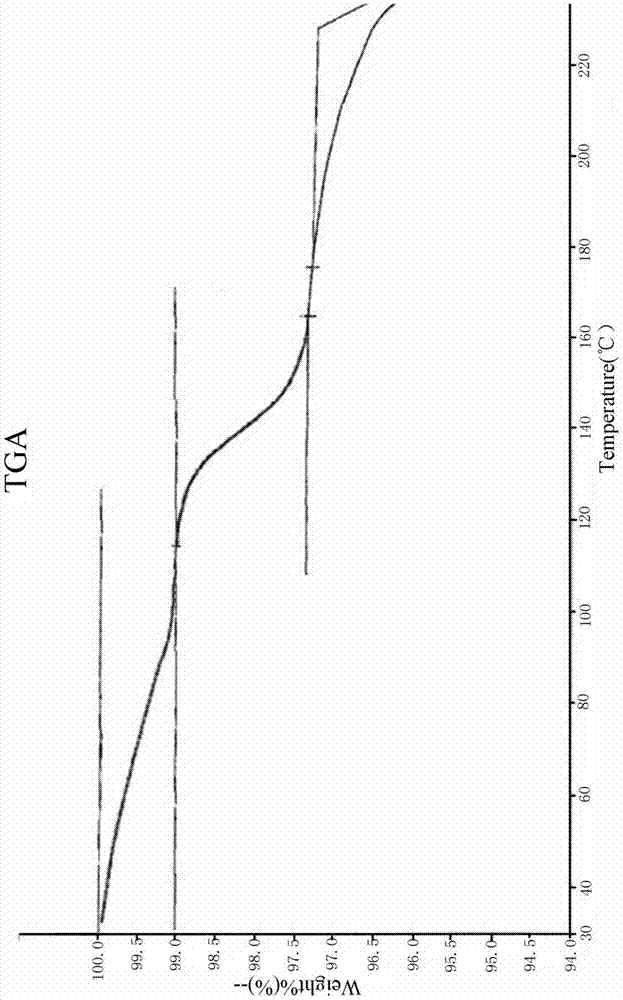
[0032] Embodiment 1: Preparation of levamlodipine maleate compound of the present invention
[0033] a. Dissolve 10 g of crude levamlodipine maleate in 200 ml of acetone, and heat to 60° C. to dissolve to obtain solution 1;
[0034] b. Under the action of an ultrasonic field (power 0.4KW), slowly add 50ml of purified water dropwise to solution 1, and turn off the ultrasonic field after adding, to obtain solution 2;
[0035] c. Lower the temperature of solution 2 to 20°C and maintain it, keep it warm and stir (40 rpm) for 2 hours, then lower the temperature to 10°C and maintain it, keep it warm for 5 hours to grow the crystal, and obtain solution 3;
[0036] d. The solution 3 was filtered, and the filter cake was dried at 50° C. for 5 hours to obtain 9.36 g of levamlodipine maleate compound of the present invention, with a yield of 92.0%.
Embodiment 2
[0037] Embodiment 2: Preparation of levamlodipine maleate compound of the present invention
[0038] a. Dissolve 10 g of crude levamlodipine maleate in 100 ml of acetone, and heat to 65° C. to dissolve to obtain solution 1;
[0039] b. Under the action of an ultrasonic field (power 0.3KW), slowly add 20ml of purified water dropwise to solution 1, and turn off the ultrasonic field after adding, to obtain solution 2;
[0040] c. Lower the temperature of solution 2 to 15°C and maintain it, keep stirring (60 rpm) for 0.5 hours, then lower the temperature to 5°C and maintain it, keep the crystal for 3 hours to obtain solution 3;
[0041] d. The solution 3 was filtered, and the filter cake was dried at 60° C. for 1 hour to obtain 9.28 g of the levoamlodipine maleate compound of the present invention, with a yield of 91.2%.
Embodiment 3
[0042] Embodiment 3: Preparation of levamlodipine maleate compound of the present invention
[0043] a. Dissolve 10 g of crude levamlodipine maleate in 300 ml of acetone, and heat to 50° C. to dissolve to obtain solution 1;
[0044] b. Under the action of an ultrasonic field (power 0.5KW), slowly add 80ml of purified water dropwise to solution 1, and turn off the ultrasonic field after adding, to obtain solution 2;
[0045] c. Lower the temperature of the solution 2 to 18°C and maintain it, keep stirring (50 rpm) for 1 hour, then lower the temperature to 7°C and maintain it, and keep the crystal for 6 hours to obtain solution 3;
[0046] d. The solution 3 was filtered, and the filter cake was dried at 45° C. for 3 hours to obtain 9.16 g of the levoamlodipine maleate compound of the present invention, with a yield of 90.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com