Multi-unit release pharmaceutical composition of amlodipine maleate and preparation method of pharmaceutical composition
A technology of amlodipine maleate and its composition is applied in the multi-unit release pharmaceutical composition of amlodipine maleate and its preparation field, which can solve the problems of low bioavailability and achieve high bioavailability, no The effect of residual organic solvent and low extrusion temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Pass the amlodipine maleate through an 80-mesh sieve, and pass the dry granulation-related auxiliary materials through an 80-mesh sieve.
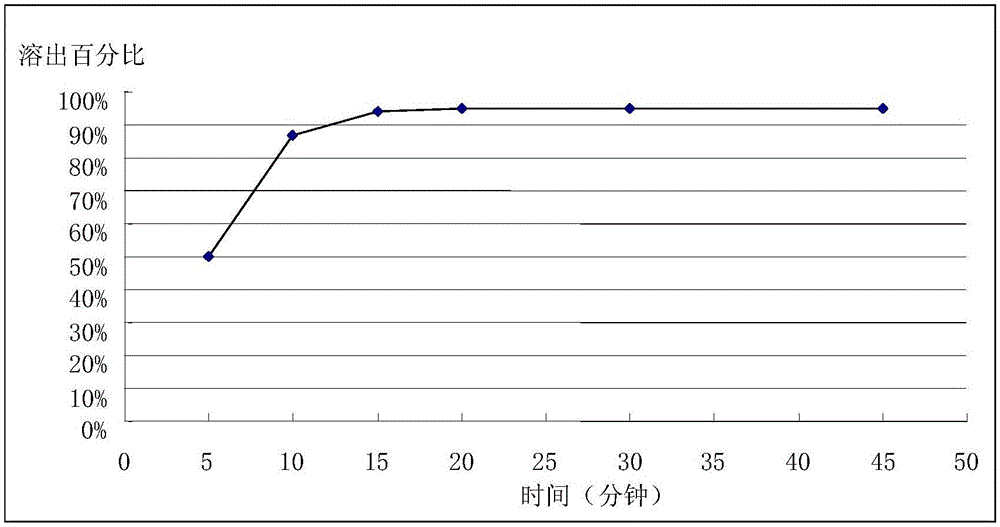
[0034] Amlodipine maleate (40%), dry granulation related excipients microcrystalline cellulose (22%), lactose (32%) croscarmellose sodium (4%), colloidal silicon dioxide ( 1%), magnesium stearate (1%) mix homogeneously, it is 5kN to set dry granulator pressure, and the feeding rod rotating speed is 10rpm, and 24 orders of pulverized granules obtain quick-release granules, measure its angle of repose and be 40.64 °, fluidity It is acceptable, but there is a phenomenon that the fill level is unstable during the capsule filling process, and the difference in tablet weight is 3.4%. Capsules are filled according to drug content. The in vitro dissolution results show that 50% of the dissolution takes 5 minutes, and more than 85% of the dissolution is reached in 10 minutes (note, the dissolution conditions in each embodiment of the applica...
Embodiment 2
[0036] Pass amlodipine maleate through an 80-mesh sieve, and pass hot-melt extrusion-related auxiliary materials through an 80-mesh sieve.
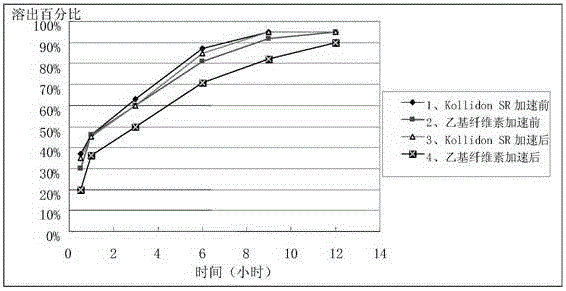
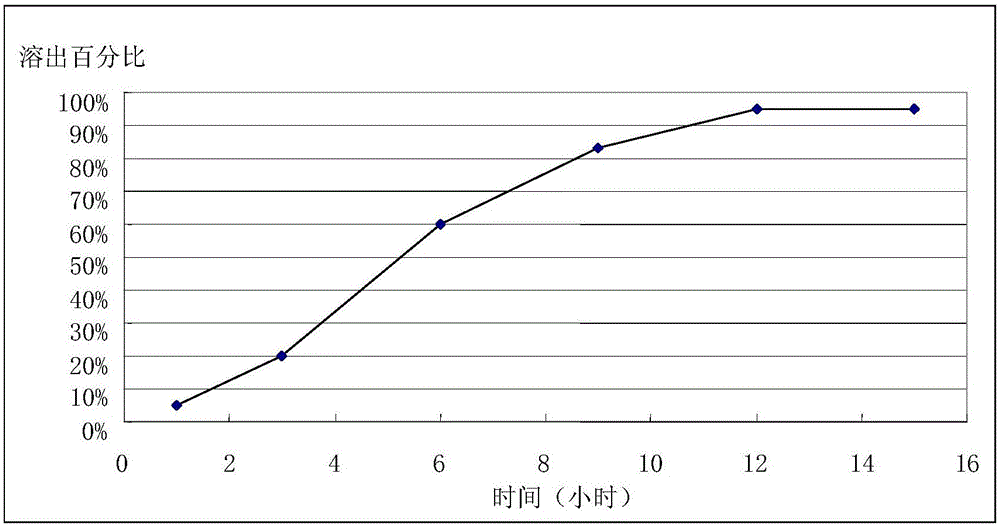
[0037] Amlodipine maleate (30%), hot-melt extrusion-related auxiliary materials polyvinyl acetate / polyvinylpyrrolidone (Kollidon SR, BASF) (59%), Span 20 (10%) anhydrous calcium hydrogen phosphate (0.5 %), sodium stearyl fumarate (0.5%) mix homogeneously, set the temperature of twin-screw extruder to be 125 ℃, when temperature rises to set temperature, add in the extruder of raw material system by feeding scale, The obtained molten extrudate is extruded in flake form by cold roll cooling, and then pulverized with a hammer mill. The number of meshes is 24 orders to obtain slow-release granules. The angle of repose is 32.13°, and the fluidity is good. During the process, the filling amount is relatively stable. It is 0.3% to measure its sheet weight difference. Capsules are filled according to drug content. The in vitro dissolution curve...
Embodiment 3
[0039]Pass amlodipine maleate through a 80-mesh sieve, and pass through an 80-mesh sieve for dry granulation-related auxiliary materials and hot-melt extrusion-related auxiliary materials.
[0040] Amlodipine maleate (40%), dry granulation related excipients microcrystalline cellulose (22%), lactose (32%) croscarmellose sodium (4%), colloidal silicon dioxide ( 1%), magnesium stearate (1%) mix homogeneously, set dry granulator pressure to be 5kN, feed rod rotating speed is 10rpm, pulverizes 24 orders of granules to obtain quick-release granules. Amlodipine maleate (30%), hot-melt extrusion related accessories polyvinyl acetate / polyvinylpyrrolidone (Kollidon SR) (59%), Span 20 (10%), anhydrous calcium hydrogen phosphate (0.5%) ), sodium stearyl fumarate (0.5%) are mixed evenly, the temperature of setting twin-screw extruder is 125 ℃, when temperature rises to set temperature, add in the extruder of raw and auxiliary material system by feeding scale, obtain The melted extrudate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com