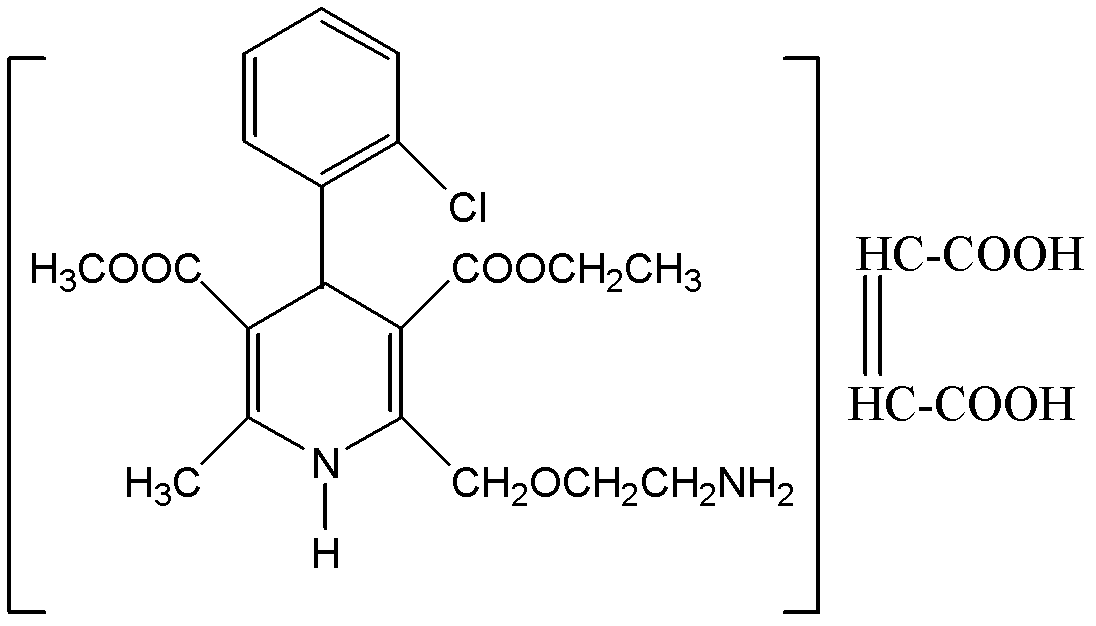
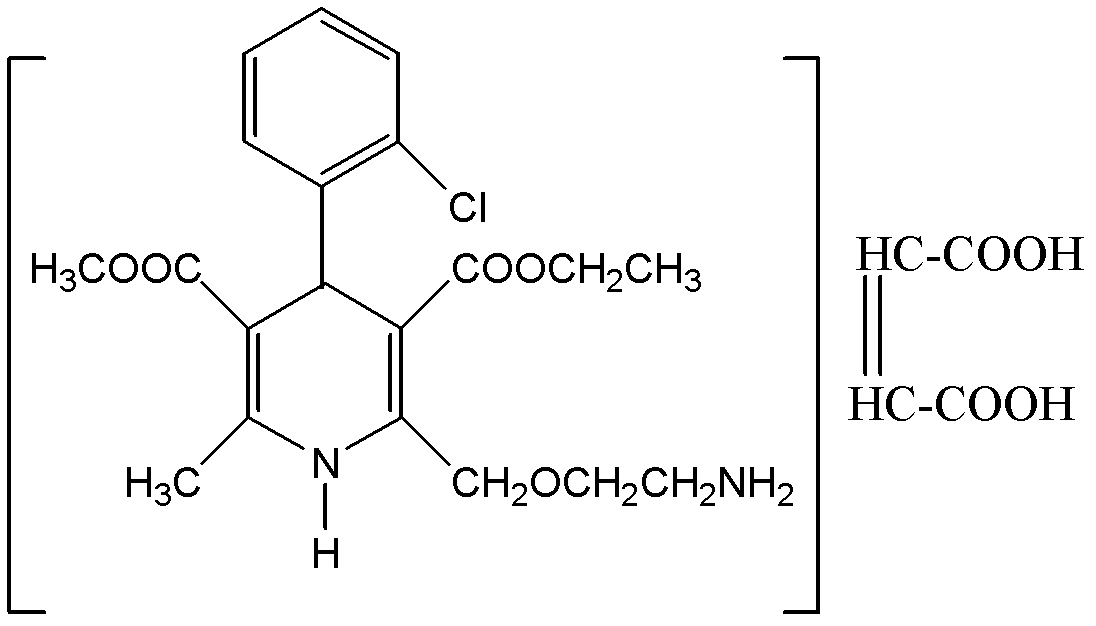
Preparation method of amlodipine maleate
A kind of technology of amlodipine maleate and amlodipine, which is applied in the field of preparation of amlodipine maleate, can solve the problems of long drying time, poor finished product crystals, difficult filtration, etc., and achieves easy vacuum filtration, reduced dosage, The effect of shortening the operation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, the preparation of amlodipine maleate salt
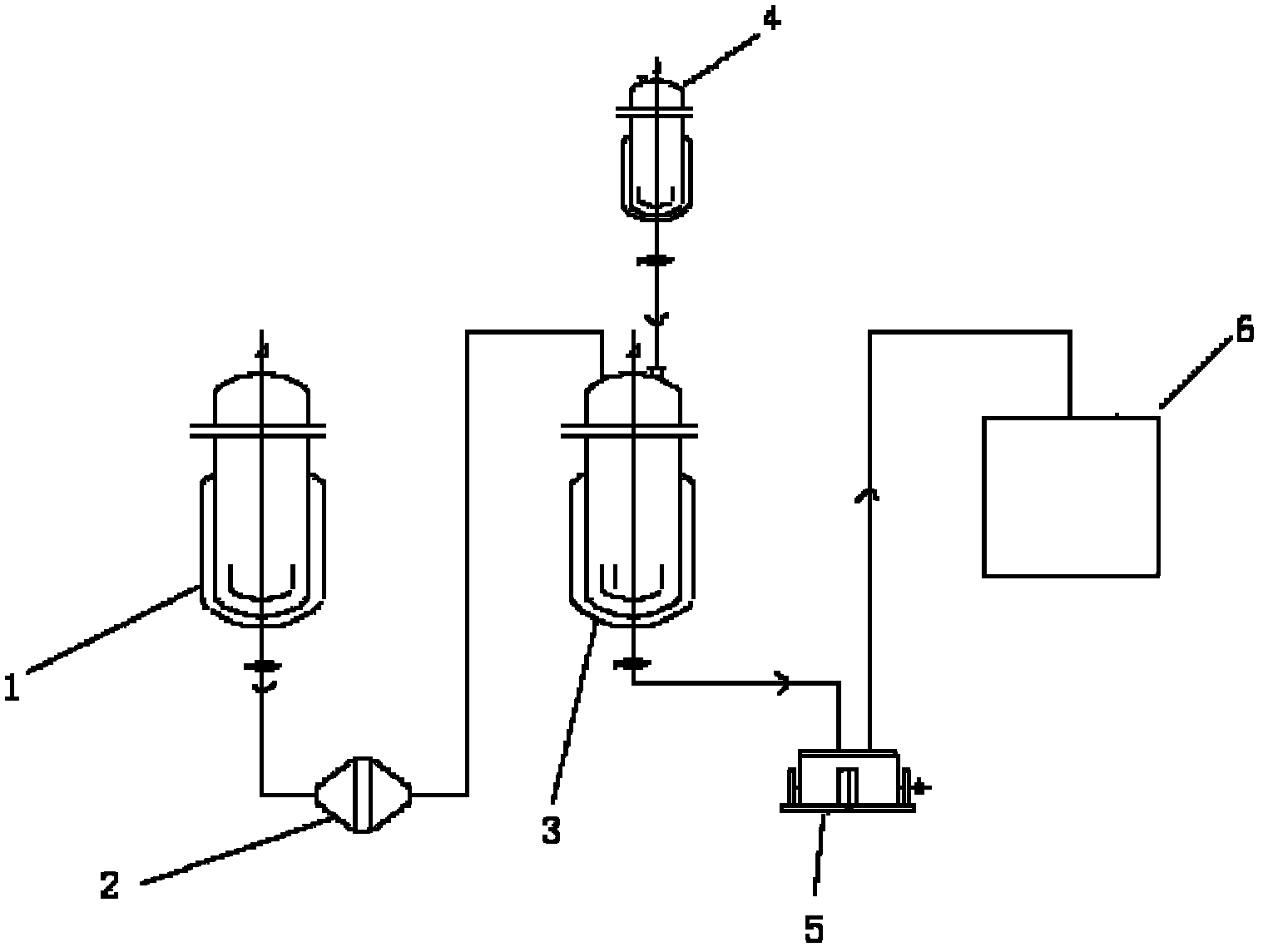
[0019] Step 1, preparation of maleic acid solution and amlodipine solution: at 35-40°C, in glass-lined tank 1, dissolve 3.0 kg of solid maleic acid into 7 liters of anhydrous methanol, and the solution Filter under pressure filter 2 into clean glass-lined tank 4; under 20-25°C, in glass-lined tank 1, dissolve 10 kg of solid amlodipine in 15 liters of anhydrous methanol . The solution is passed through a pressure filter 2 into a clean glass-lined tank 3 by means of pressure filtration.
[0020] Step 2, the generation of amlodipine maleate salt: the maleic acid solution prepared in the previous step is slowly added dropwise into the glass-lined tank 3 through the glass-lined tank 4, and the temperature in the reactor is kept at 20-25 ° C. After the addition was complete, the mixture was kept at 20-30°C and stirred for 3 hours, then slowly cooled to 3°C, and the mixture was allowed to stand for 12 hours.
[0021...
Embodiment 2
[0023] Embodiment two, the preparation of amlodipine maleate salt
[0024] Step 1, preparation of maleic acid solution and amlodipine solution: at 35-40°C, in glass-lined tank 1, dissolve 3.5 kg of solid maleic acid into 7 liters of anhydrous methanol, and the solution Filter into a clean glass-lined tank 4 by pressure filter 2 in the manner of pressure filtration; 15 kilograms of solid amlodipine are all dissolved in 15 liters of anhydrous methanol in the glass-lined tank 1 under the condition of 20-25 ° C, and the solution Filter 2 into a clean glass-lined tank 3 through a press filter by means of pressure filtration.
[0025] Step 2, the generation of amlodipine maleate salt: the maleic acid solution prepared in the previous step is slowly added dropwise into the glass-lined tank 3 through the glass-lined tank 4, and the temperature in the reactor is kept at 20-25 ° C. After the addition was complete, the mixture was kept and stirred at 25-40°C for 2 hours, then slowly coo...
Embodiment 3
[0028] Embodiment three, the preparation of amlodipine maleate salt
[0029] Step 1, preparation of maleic acid solution and amlodipine solution: 1.4 kg of solid maleic acid is completely dissolved in 7 liters of anhydrous methanol in glass-lined tank 1 at 35-40 ° C, and the solution is added to The method of pressure filtration is filtered into a clean glass-lined tank 4 through a pressure filter 2; 5 kg of solid amlodipine is completely dissolved in 15 liters of anhydrous methanol in a glass-lined tank 1 at 20-25 ° C, and the solution is added with Press Filtration Method Filter through a press filter 2 into a clean glass-lined tank 3 .
[0030] Step 2, the generation of amlodipine maleate salt: the maleic acid solution prepared in the previous step is slowly added dropwise into the glass-lined tank 3 through the glass-lined tank 4, and the temperature in the reactor is kept at 20-25 ° C. After the addition was complete, the mixture was kept and stirred at 25-50°C for 5 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com