Preparation method of amlodipine besylate tablets
A kind of technology of amlodipine besylate tablet and formula, applied in the field of preparation of amlodipine besylate tablet, can solve the problems of poor dissolution of tablet amlodipine, unstable product quality and the like, and achieve great clinical application value , suitable disintegration time, good fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
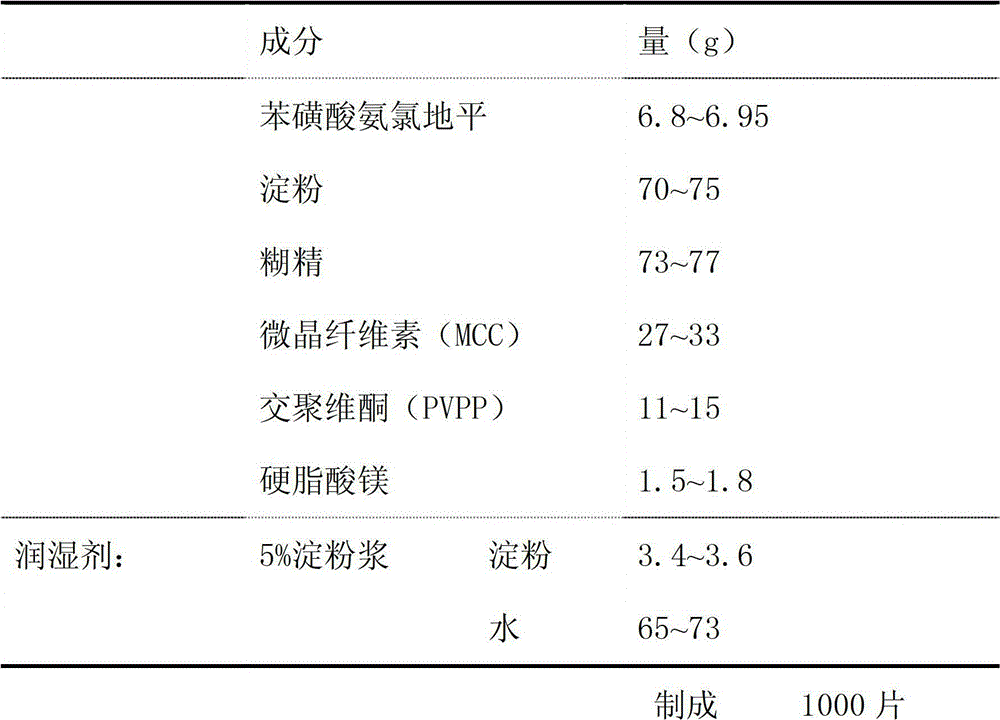
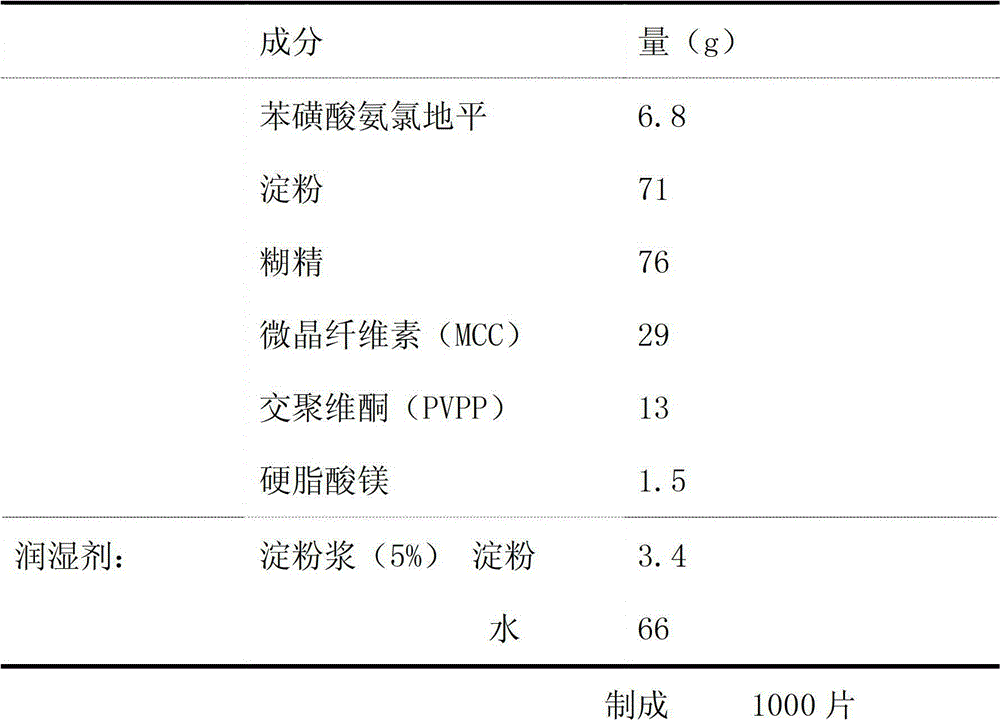
[0025] Embodiment 1 Amlodipine besylate sheet preparation
[0026] formula:
[0027]
[0028] method:
[0029] (1) Pass each component through an 80-mesh sieve, place at 50°C for 20-40 minutes, and dry for later use;
[0030] (2) After mixing amlodipine besylate, microcrystalline cellulose, and crospovidone evenly, use 5% starch slurry to make a soft material, pass through a 18-mesh sieve, and granulate at 50°C for 20-40 minutes. Dry, and then sieve the 18-mesh granule to obtain granule I;
[0031] (3) After mixing starch and dextrin evenly, use 5% starch slurry to make soft material, pass through a 18-mesh sieve to granulate, dry at 50°C, and then granulate through a 18-mesh sieve to obtain Granule II;
[0032] (4) Mix granule I and granule II, add magnesium stearate, and mix evenly to obtain the raw material of amlodipine besylate preparation;
[0033] (5) Take the raw material of amlodipine besylate preparation and press it into tablets with a tablet machine (ф8mm sh...
Embodiment 2
[0036] formula:
[0037]
[0038] The preparation method is the same as in Example 1.
Embodiment 3
[0040] formula:
[0041]
[0042]
[0043] The preparation method is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com