Method for preparing compound pseudo-ginseng film coated tablet
A technology of compound notoginseng and film-coated tablets, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problem that the content of active ingredients cannot meet the minimum requirements of the standard and the disintegration of sugar-coated tablets The time limit exceeds the limit, sugar-coated tablets are easy to absorb moisture, etc., to achieve the effects of saving coating materials, accelerating release and absorption speed, and easy disintegration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
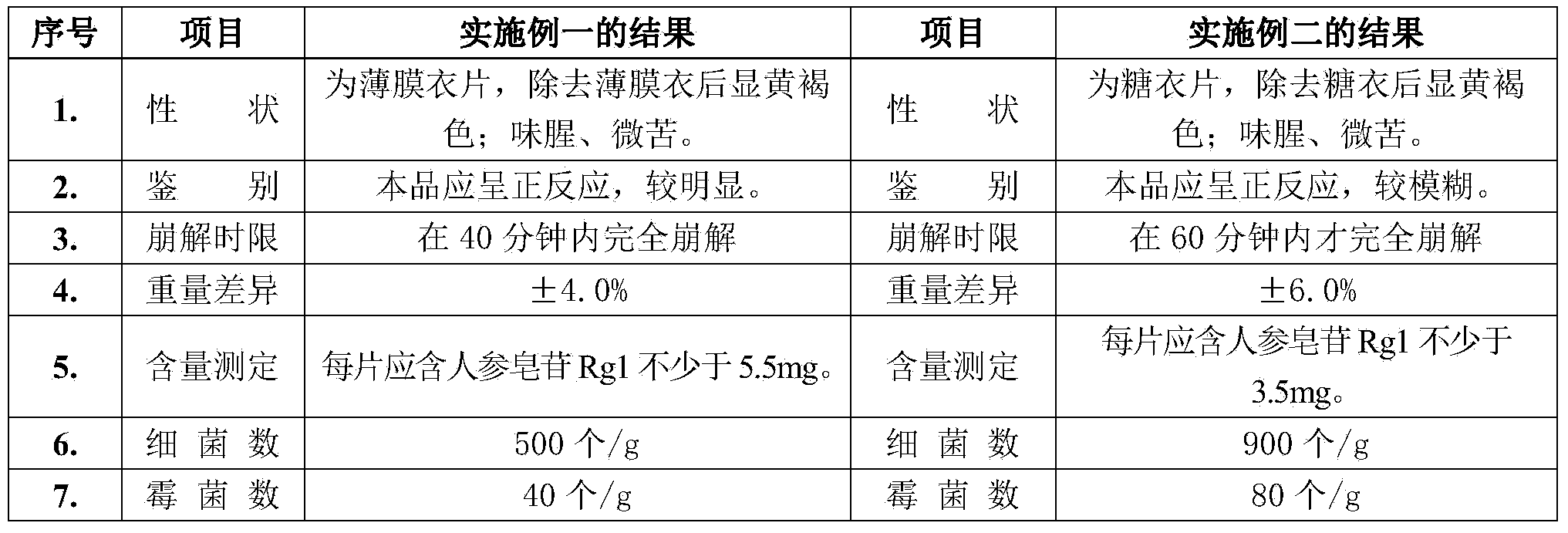
[0035] Embodiment one (compound notoginseng tablet prepared by film coating provided by the invention)
[0036] Raw materials include: Panax notoginseng 45.57Kg, Angelica dahurica 10.63Kg, Wood beetle 10.63Kg, Ligusticum chuanxiong 10.63Kg, Angelica 10.63Kg, Safflower 10.63Kg, Frankincense 10.63Kg, Myrrh 10.63Kg; Excipients include: Magnesium stearate 4.8Kg, Sodium carboxymethylcellulose (CMC) 0.72Kg; film coating powder 12Kg.
[0037] The steps of preparing the compound Sanqi film-coated tablet by adopting the above-mentioned crude drug, auxiliary material and film-coating powder are as follows in sequence:
[0038] (1) Pulverization: According to the above formula, take the processed medicinal materials of the 8 raw materials and put them in a microwave drying sterilizer for drying and sterilization at 70° C. until the water content is ≤4.0%, take them out, pulverize them into fine powders, and 80-mesh sieve, put into a double-layer clean plastic bag, and attach a material ...
Embodiment 2
[0049] Embodiment two (compound notoginseng tablet of traditional craft bag sugar coating)
[0050] The raw materials include: Panax notoginseng 45.57Kg, Angelica dahurica 10.63Kg, wood beetle 10.63Kg, Chuanxiong 10.63Kg, angelica 10.63Kg, safflower 10.63Kg, frankincense 10.63Kg, myrrh 10.63Kg.
[0051] (1) Pulverization: According to the above formula, the processed medicinal materials of the 8 herbs are respectively pulverized into fine powder, passed through an 80-mesh sieve, and attached with a material stick.
[0052] (2) Granulation: Add the extract powder into the mixer and mix evenly, add water and stir for 10 minutes to obtain soft material; put the soft material into the granulator to granulate; use 12 mesh iron screen to make the wet granules, mix the wet The particles are transferred to a hot air oven and dried at 115°C until the water content is less than 7%.
[0053] (3) Tablet compression: Use a 9 mm diameter deep arc die to compress the granules into tablets i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com