Synthesis technology of amlodipine maleate
A kind of technology of amlodipine maleate and synthesis process, applied in the direction of organic chemistry and the like, can solve the problems of many by-products and impurities, and achieve the effects of reducing the content of impurities, reducing the cost of products, and strong controllability of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
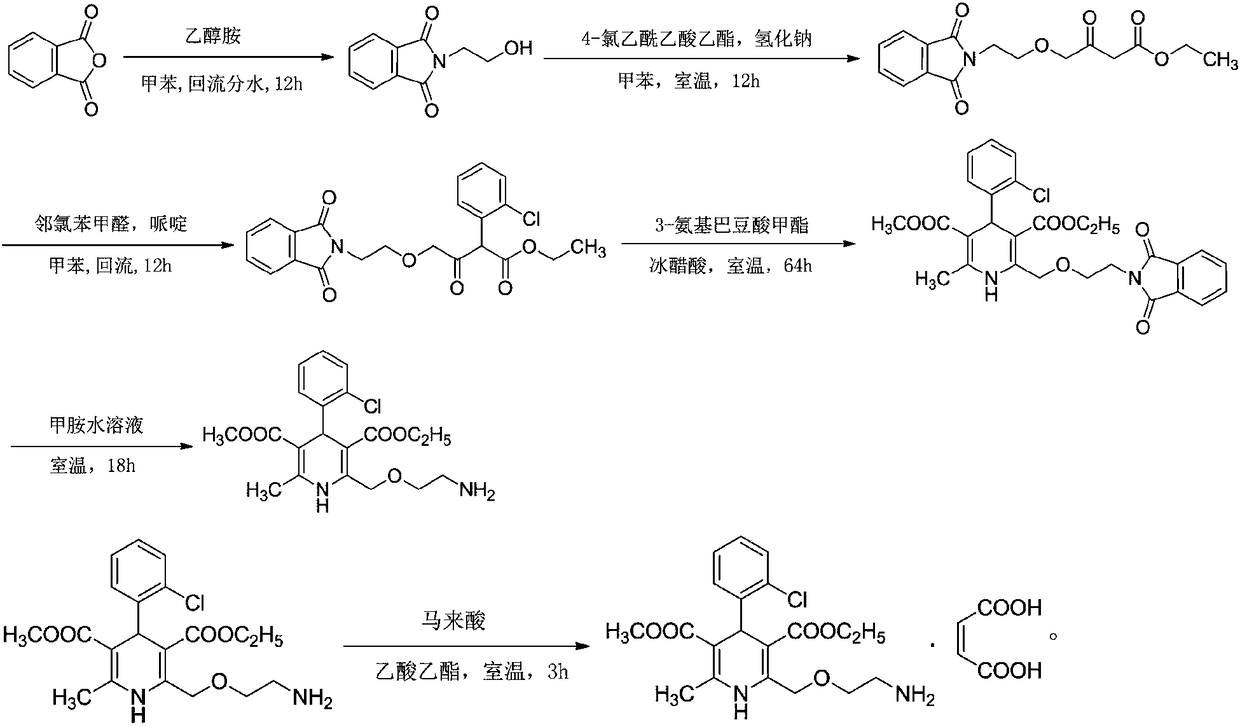
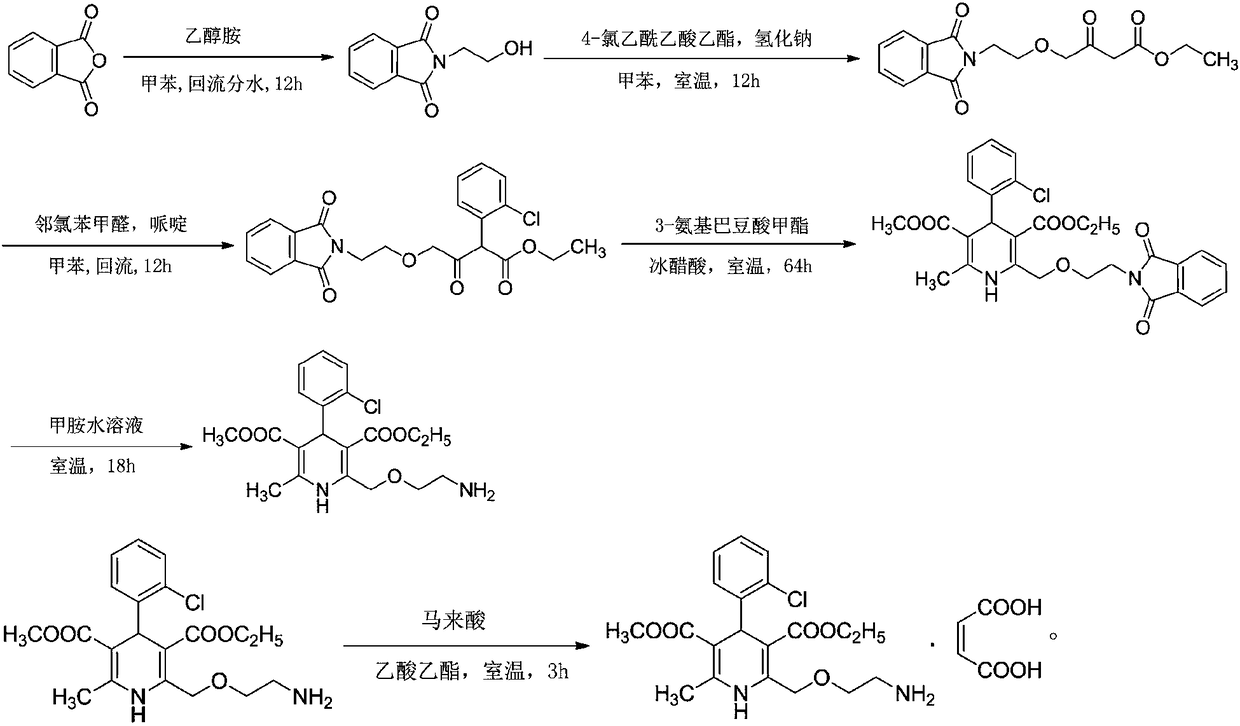
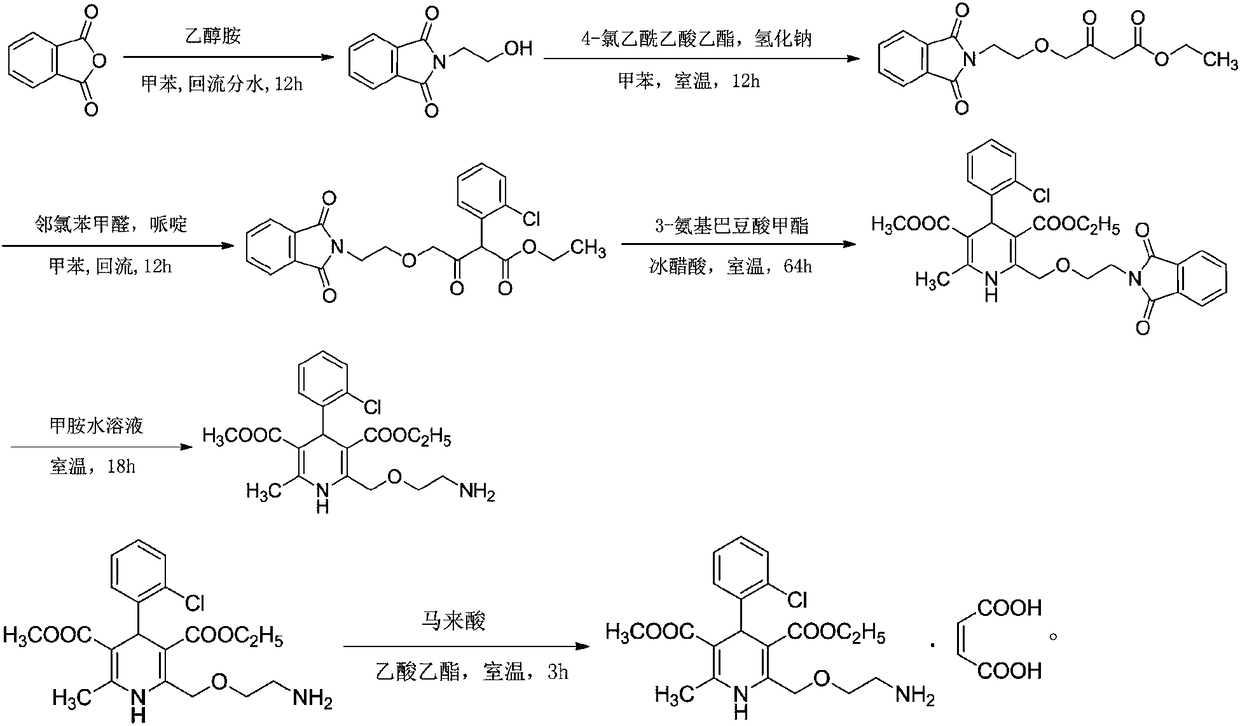
[0034] Embodiment 1: a kind of synthetic technique of amlodipine maleate comprises the following steps:
[0035] Step 1, the preparation of N-(2-hydroxyethyl)-phthalimide:
[0036] In the reaction tank, put 133kg of toluene, add 40kg of phthalic anhydride under stirring condition, raise the temperature to 60°C, add 16.4kg of ethanolamine dropwise, after adding, raise the temperature to 110°C, reflux and dehydrate for more than 12 hours, cool down to 15°C, and centrifugally filter. Wash the filter residue with toluene, dry it, dry it at 65°C and a vacuum of -0.1MPa, cool it down to normal temperature, and discharge to obtain 51kg of N-(2-hydroxyethyl)-phthalimide ;
[0037] Step 2, the preparation of 4-[(2-phthalimide) ethoxy] ethyl acetoacetate:
[0038]Put 350kg of toluene into the reaction tank, stir and heat up, and distill the toluene. After the liquid in the water separator is full, keep reflux and water separation for 60 minutes, and take samples to measure the water s...
Embodiment 2
[0053] Embodiment 2: a kind of synthetic technique of amlodipine maleate, the difference with embodiment 1 is that step one is: in reaction tank, drop into 133kg toluene, add 40kg phthalic anhydride under stirring condition, be warming up to 60 ℃, add 16.4kg of ethanolamine dropwise, heat up to 110℃ after adding, reflux dehydration for more than 12 hours, cool down to 15℃, centrifugal filter, wash filter residue with toluene, spin dry, at 70℃, vacuum degree is -0.1MPa Drying under low temperature, cooling to normal temperature, discharging to obtain 51 kg of N-(2-hydroxyethyl)-phthalimide; the yield of amlodipine maleate is 91.6%, and the purity is 99.4%.
Embodiment 3
[0054] Embodiment 3: A kind of synthetic technique of amlodipine maleate, the difference with embodiment 1 is that step 2 is: in reaction tank, drop into 350kg toluene, stir and heat up, distill toluene, wait in water separator After the liquid is full, keep reflux and divide water for 60 minutes, take a sample to measure the water divide, when the water content of the reflux liquid is less than or equal to 0.2%, change the reflux to distillation, distill 45kg of toluene (as the dilution solvent of ethyl 4-chloroacetoacetate), stir Cool down, when the temperature in the tank drops to 45°C, fill the reaction tank with nitrogen, continue to cool down to 0°C, add 40kg of N-(2-hydroxyethyl)-phthalimide under stirring, and then add 20kg Sodium hydride, after adding, cover the tank tightly, stir at 0°C, pump 38.4kg of ethyl 4-chloroacetoacetate and 45kg of toluene into the metering tank and mix them evenly, and pour them into the reaction tank at 0°C Add the toluene solution of ethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com