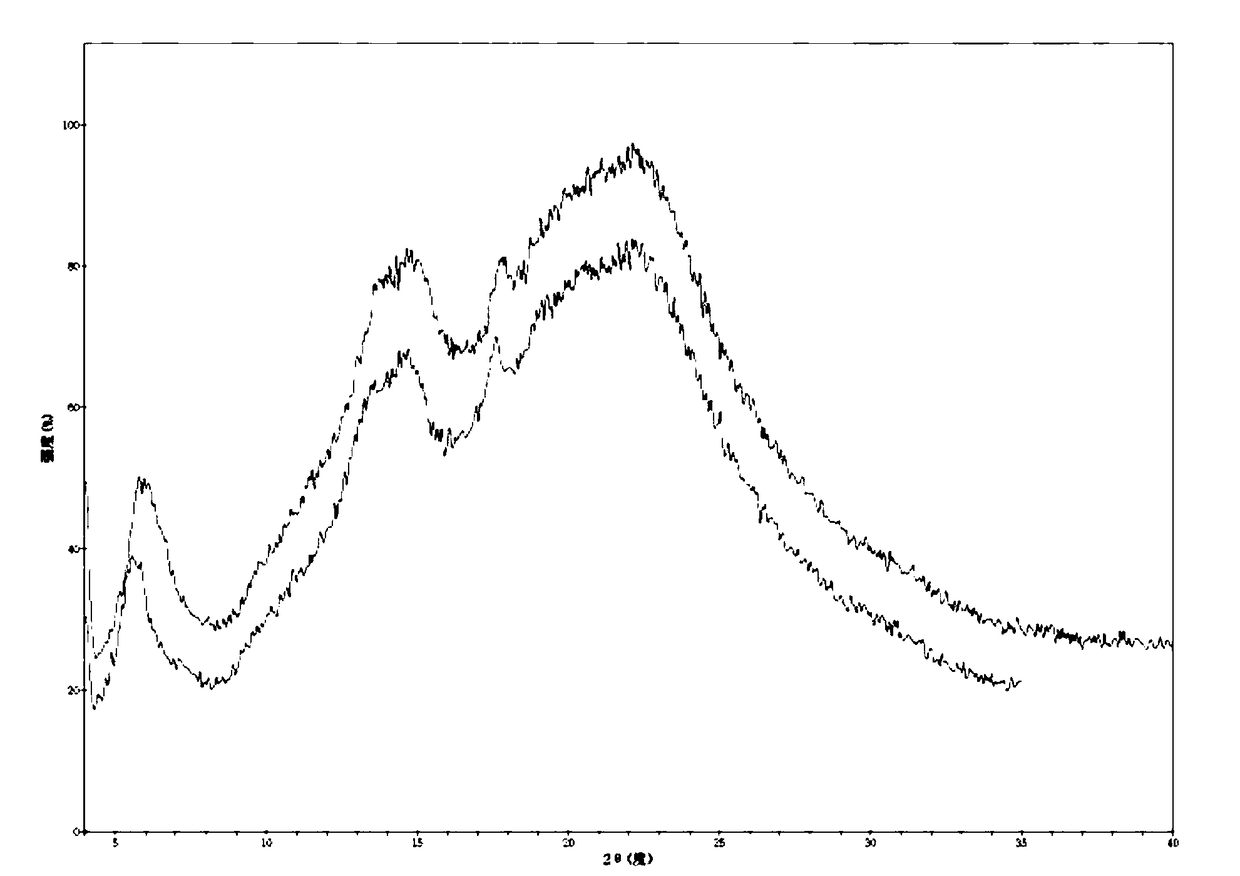
Preparation method of valsartan and amlodipine tablets and valsartan and amlodipine tablets
A technology of valsartan amlodipine tablet and amlodipine besylate, applied in the field of biomedicine, can solve the problems of different dissolution and dissimilar dissolution of valsartan amlodipine tablet, and achieves easy operation and low production cost , the effect of enhanced adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] A valsartan amlodipine tablet, consisting of the following raw materials in parts by weight: valsartan (batch 2) 75, amlodipine besylate 5, microcrystalline cellulose 50, crospovidone 18, colloidal Silicon dioxide 1.2, magnesium stearate 4.0.
[0073] Described valsartan amlodipine sheet is made by following method:
[0074] (1) Valsartan and amlodipine besylate are adopted pulverizer (LFP-1000A high-speed multifunctional pulverizer, Leif brand), and after pulverizing 1min at a rotating speed of 25000rpm, a premixed material is obtained, and then mixed with microcrystalline cellulose , crospovidone, colloidal silicon dioxide and 60% of the total magnesium stearate mass are mixed to obtain the initial mixture;
[0075] (2) Dry granulation: Take the primary mixture of step (1) and use a dry granulator (DP-5 dry granulator, Shenzhen Xinyite), and roll it into a strip, integrated crushing, granulation (DP -5 dry granulator integrated conical rotary granulator), the size o...
Embodiment 2
[0080] A valsartan amlodipine tablet, consisting of the following raw materials in parts by weight: valsartan (batch 2) 80, amlodipine besylate 6.94, microcrystalline cellulose 54.06, crospovidone 20, colloidal Silicon dioxide 1.5, magnesium stearate 4.5.
[0081] Described valsartan amlodipine sheet is made by following method:
[0082] (1) Valsartan and amlodipine besylate are adopted pulverizer (LFP-1000A high-speed multifunctional pulverizer, Leif brand), and after pulverizing 1min at a rotating speed of 25000rpm, a premixed material is obtained, and then mixed with microcrystalline cellulose , crospovidone, colloidal silicon dioxide and 60% of the total magnesium stearate mass are mixed to obtain the initial mixture;
[0083] (2) Dry granulation: Take the primary mixture of step (1) and use a dry granulator (DP-5 dry granulator, Shenzhen Xinyite), and roll it into a strip, integrated crushing, granulation (DP -5 dry granulator integrated conical rotary granulator), the ...
Embodiment 3
[0089] Prescription, technology are identical with embodiment 2, just valsartan batch is different.
[0090] A valsartan amlodipine tablet, consisting of the following raw materials in parts by weight: valsartan (batch 1) 80, amlodipine besylate 6.94, microcrystalline cellulose 54.06, crospovidone 20, colloidal Silicon dioxide 1.5, magnesium stearate 4.5.
[0091] Described valsartan amlodipine sheet is made by following method:
[0092] (1) Valsartan and amlodipine besylate are adopted pulverizer (LFP-1000A high-speed multifunctional pulverizer, Leif brand), and after pulverizing 1min at a rotating speed of 25000rpm, a premixed material is obtained, and then mixed with microcrystalline cellulose , crospovidone, colloidal silicon dioxide and 60% of the total magnesium stearate mass are mixed to obtain the initial mixture;
[0093] (2) Dry granulation: Take the primary mixture of step (1) and use a dry granulator (DP-5 dry granulator, Shenzhen Xinyite), and roll it into a str...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com