Valsartan amlodipine pharmaceutical composition and preparation method thereof
A technology of amlodipine besylate and composition, which is applied in the field of pharmaceutical composition containing valsartan and amlodipine besylate and its preparation, and can solve the problems of low bioavailability, large difference in tablet weight, and drug disintegration. Solve the problems such as unsatisfactory dissolution and dissolution, achieve the effect of simple production process, improve dissolution, and reduce investment in equipment and workshops
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
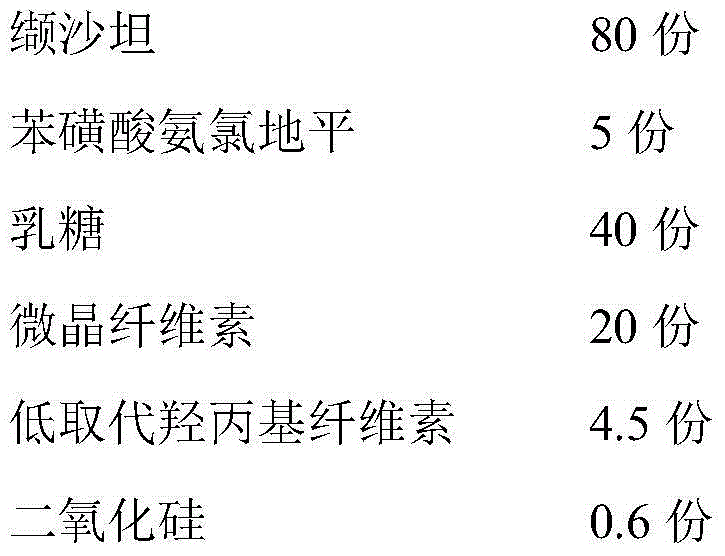
[0074] 1. Prescription
[0075] Tablet prescription:
[0076]
[0077] Coating Solution Prescription:
[0078] Opadry II 5g
[0079] Purified water 28ml
[0080] 2. Preparation process
[0081] (1) Pass the valsartan raw material and the amlodipine besylate raw material through an 80-mesh sieve respectively.
[0082] (2) Bake lactose, microcrystalline cellulose, croscarmellose sodium, silicon dioxide and sodium stearyl fumarate at 80°C for 2 hours, and pass through a 60-mesh sieve.
[0083] (3) Weighing valsartan, lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, silicon dioxide and sodium stearyl fumarate according to the prescription amount, and fully mixing to obtain a mixed powder.
[0084] (4) Weigh the prescribed amount of amlodipine besylate and mix with the above-mentioned mixed powder according to the method of equal increase.
[0085] (5) Determine the content of the main drug, calculate the weight of the tablet, press the tablet ...
Embodiment 2
[0089] 1. Prescription
[0090] Tablet prescription:
[0091]
[0092] Coating Solution Prescription:
[0093] Opadry II 5g
[0094] Purified water 28ml
[0095] 2. Preparation process
[0096] (1) Pass the valsartan raw material and the amlodipine besylate raw material through a 100-mesh sieve respectively.
[0097] (2) Bake lactose, microcrystalline cellulose, croscarmellose sodium, silicon dioxide and sodium stearyl fumarate at 60°C for 3 hours, and pass through a 60-mesh sieve.
[0098] (3) Weighing valsartan, lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, silicon dioxide and sodium stearyl fumarate according to the prescription amount, and fully mixing to obtain a mixed powder.
[0099] (4) Weigh the prescribed amount of amlodipine besylate and mix with the above-mentioned mixed powder according to the method of equal increase.
[0100] (5) Determine the content of the main drug, calculate the weight of the tablet, press the tablet ...
Embodiment 3
[0104] 1. Prescription
[0105] Tablet prescription:
[0106]
[0107] Made into 1000 pieces
[0108] Coating Solution Prescription:
[0109] Opadry II 5g
[0110] Purified water 22ml
[0111] 2. Preparation process
[0112] (1) Pass the valsartan raw material and the amlodipine besylate raw material through a 100-mesh sieve respectively.
[0113] (2) Bake lactose, microcrystalline cellulose, croscarmellose sodium, silicon dioxide and sodium stearyl fumarate at 70°C for 3 hours, and pass through an 80-mesh sieve.
[0114] (3) Weighing valsartan, lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, silicon dioxide and sodium stearyl fumarate according to the prescription amount, and fully mixing to obtain a mixed powder.
[0115] (4) Weigh the prescribed amount of amlodipine besylate and mix with the above-mentioned mixed powder according to the method of equal increase.
[0116] (5) Determine the content of the main drug, calculate the weight...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com