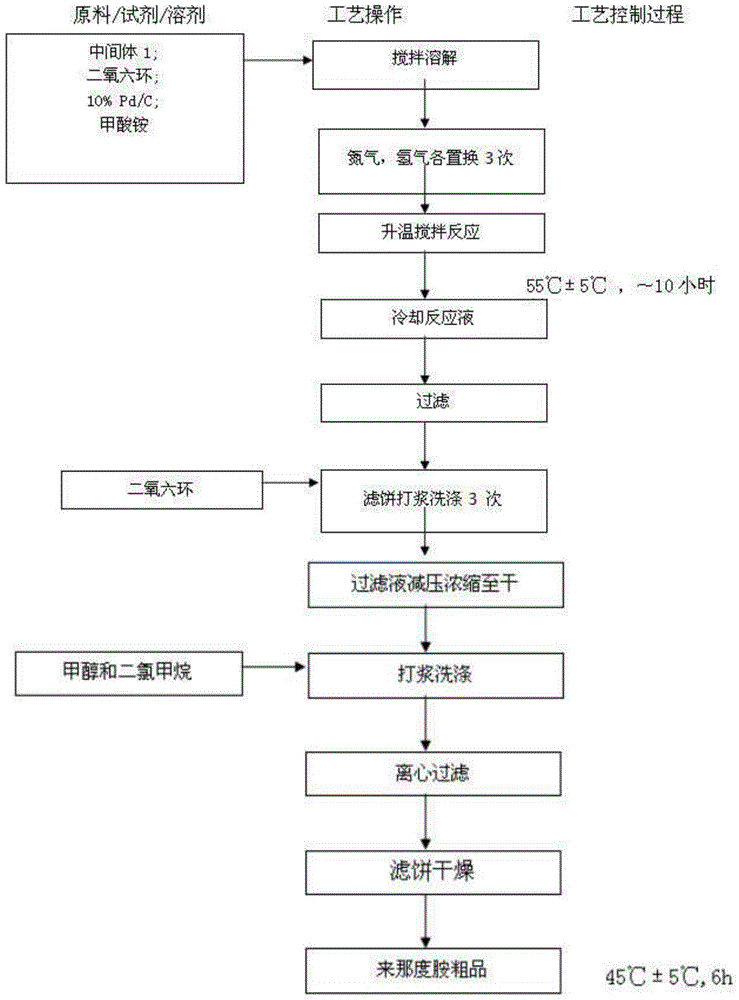
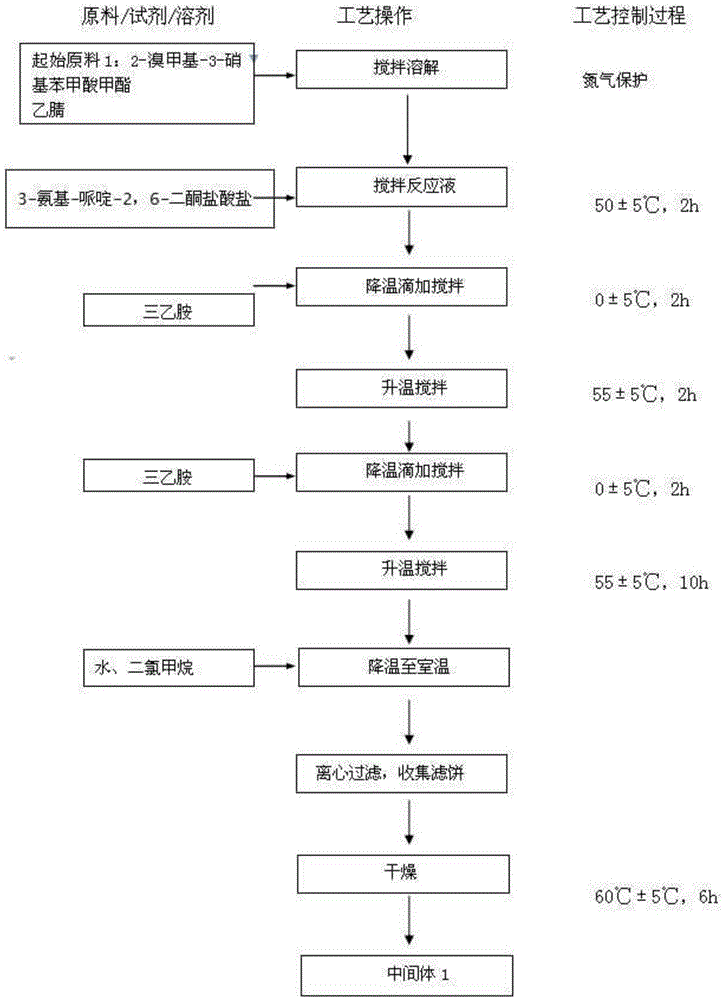
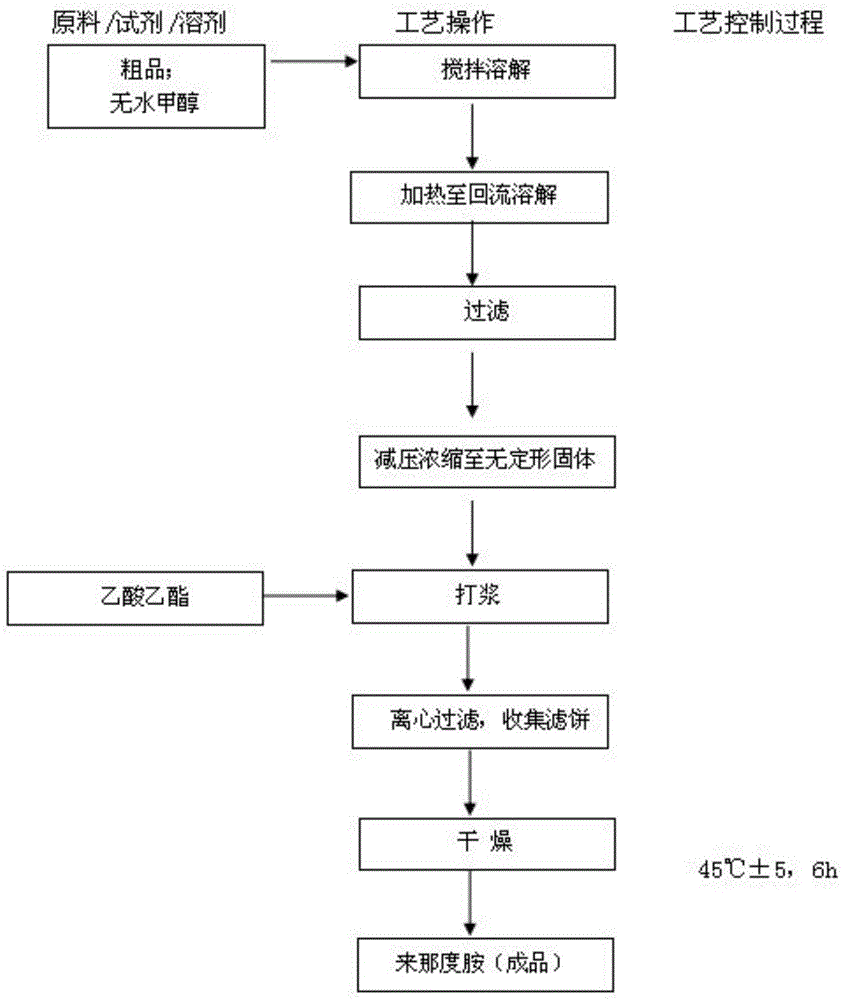
Lenalidomide composition tablets and preparation method thereof
A technology for lenalidomide and composition, which is applied in the field of lenalidomide composition tablet and preparation thereof, can solve the problem that the granulation porosity reproducibility is affected by many factors, the drug dissolution stability is greatly affected, and the The problems of granulation and drying take a long time, and the preparation process is simple and easy to operate, the content of moisture and impurities is low, and the crystal form is stable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A lenalidomide composition tablet, the composition of which comprises by weight percentage: 10% of lenalidomide crystals, 40% of lactose, 30% of microcrystalline cellulose, 2% of konjac glucomannan, β-cyclodextrin 10%, sodium carboxymethyl starch 6%, sodium stearyl fumarate 0.5%, powdered cellulose 1.5%.
[0037] A preparation method of the above-mentioned lenalidomide composition tablet, comprising the following steps:
[0038] Step 1, mixing lenalidomide crystals with lactose, microcrystalline cellulose, and sodium carboxymethyl starch at a rotating speed of 500 r / min, pulverizing, and passing through a 100-mesh sieve to obtain 100-mesh mixture particles;
[0039] Step 2. Add konjac glucomannan and β-cyclodextrin to purified water and ethanol with a volume ratio of 1:2, stir evenly to obtain a granulation liquid, add the granulation liquid to the mixture particles, stir evenly, filter, Granulate to obtain wet granules, dry the wet granules at 60°C to obtain dry granu...
Embodiment 2
[0043] A lenalidomide composition tablet, the composition of which comprises by weight percentage: 15% of lenalidomide crystals, 34% of lactose, 28% of microcrystalline cellulose, 3% of konjac glucomannan, β-cyclodextrin 12%, sodium carboxymethyl starch 6%, sodium stearyl fumarate 0.6%, powdered cellulose 1.4%.
[0044] A preparation method of the above-mentioned lenalidomide composition tablet, comprising the following steps:
[0045] Step 1, mixing lenalidomide crystals with lactose, microcrystalline cellulose, and sodium carboxymethyl starch at a rotating speed of 300 r / min, pulverizing, and passing through a 200-mesh sieve to obtain 200-mesh mixture particles;
[0046] Step 2. Add konjac glucomannan and β-cyclodextrin to purified water and ethanol with a volume ratio of 1:4, stir evenly to obtain a granulation liquid, and input the granulation liquid into a stainless steel nozzle with voltage applied, Apply high-voltage static electricity on the stainless steel nozzle thr...
Embodiment 3
[0050] A lenalidomide composition tablet, the composition of which comprises by weight percentage: 16% of lenalidomide crystals, 31% of lactose, 28% of microcrystalline cellulose, 3.7% of konjac glucomannan, β-cyclodextrin 12%, sodium carboxymethyl starch 8%, sodium stearyl fumarate 0.8%, powdered cellulose 0.5%.
[0051] A preparation method of the above-mentioned lenalidomide composition tablet, comprising the following steps:
[0052] Step 1. Add crude lenalidomide and polymethyl methacrylate with a weight ratio of 1:4 to the dimethyl sulfoxide solution, stir until completely dissolved to obtain a mixture solution, and input the mixture solution to a voltage-applied In the stainless steel nozzle, apply high-voltage static electricity on the stainless steel nozzle through a high-voltage power supply, spray the mixture solution from the stainless steel nozzle and spray it into the receiving device containing isopropanol and water with a volume ratio of 1:1 at 60 ° C, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com