Tenofovir alafenamide fumarate tablet, preparation method thereof and detection method of related substances
A technology of tenofovir fumarate and tenofol fumarate, which is applied in the field of tenofovir fumarate and tenofovir fumarate tablets and its preparation, can solve the problems of slow product dissolution, cumbersome process, poor consistency and the like , to achieve the effects of high drug stability, volume reduction, and narrow particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
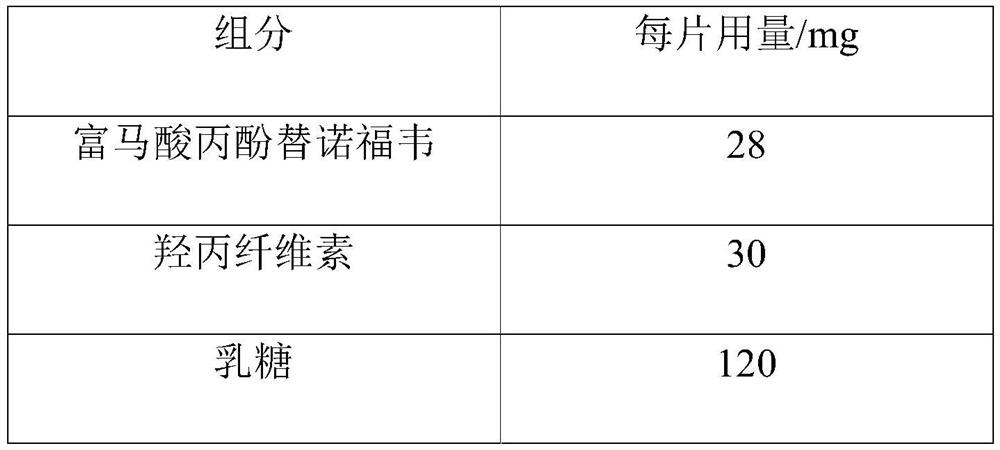
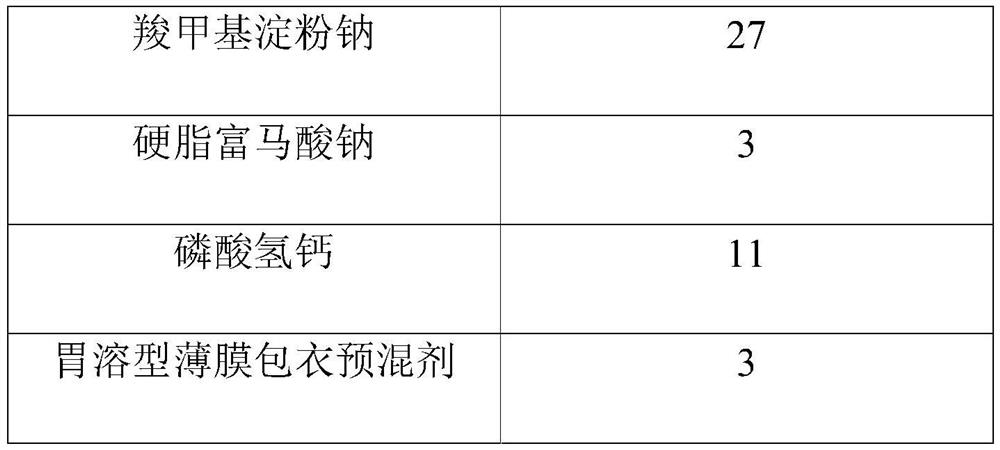
[0042] The present embodiment provides a tenofovir alafenamide fumarate tablet, and the prescription dosage is as shown in the table below:
[0043]
[0044]
[0045] The preparation method of above-mentioned tenofovir alafenamide fumarate tablet is as follows:
[0046]The tenofovir alafenamide fumarate raw material is crushed through a 100-mesh sieve for later use, and other auxiliary materials are passed through an 80-mesh sieve for later use. According to the principle of the main drug after the auxiliary material first, each component is weighed according to the prescription amount, and the weighed tenofovir alafenamide fumarate, 65wt% prescription amount hydroxypropyl cellulose, lactose, sodium carboxymethyl starch , calcium hydrogen phosphate and 68wt% prescription amount of sodium stearyl fumarate are mixed evenly, and the fluidized bed granulation is carried out. The temperature of the air inlet is controlled at 20 ° C. After the granulation is completed, add the...
Embodiment 2
[0050] The present embodiment provides a tenofovir alafenamide fumarate tablet, and the prescription dosage is as shown in the table below:
[0051]
[0052]
[0053] The preparation method of above-mentioned tenofovir alafenamide fumarate tablet is as follows:
[0054] The tenofovir alafenamide fumarate raw material is crushed through a 100-mesh sieve for later use, and other auxiliary materials are passed through an 80-mesh sieve for later use. According to the principle of the main drug after the auxiliary material first, each component is weighed according to the prescription amount, and the weighed tenofovir alafenamide fumarate, 70wt% prescription amount hydroxypropyl cellulose, lactose, sodium carboxymethyl starch , calcium hydrogen phosphate and 65wt% prescription amount of sodium stearyl fumarate are mixed evenly, and the fluidized bed granulation is carried out. The temperature of the air inlet is controlled at 30 ° C. After the granulation is completed, add th...
Embodiment 3
[0058] The present embodiment provides a tenofovir alafenamide fumarate tablet, and the prescription dosage is as shown in the table below:
[0059] components Dosage per tablet / mg tenofovir alafenamide fumarate 28 Hypromellose 31 lactose 106 Sodium carboxymethyl starch 15 Sodium stearyl fumarate 6 Calcium hydrogen phosphate 11 Gastric film coating premix 10
[0060] The preparation method of above-mentioned tenofovir alafenamide fumarate tablet is as follows:
[0061] The tenofovir alafenamide fumarate raw material is crushed through a 100-mesh sieve for later use, and other auxiliary materials are passed through an 80-mesh sieve for later use. According to the principle of the main drug after the auxiliary material first, each component is weighed according to the prescription amount, and the weighed tenofovir alafenamide fumarate, 68wt% prescription amount hydroxypropyl cellulose, lactose, sodium carboxymethyl starch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Film diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com