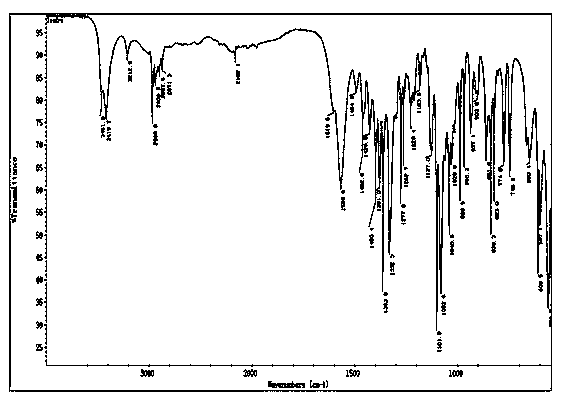
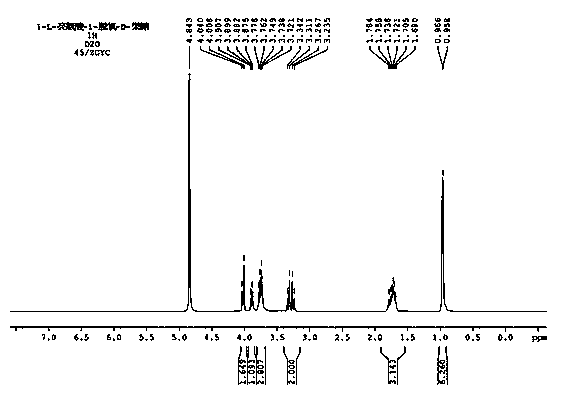
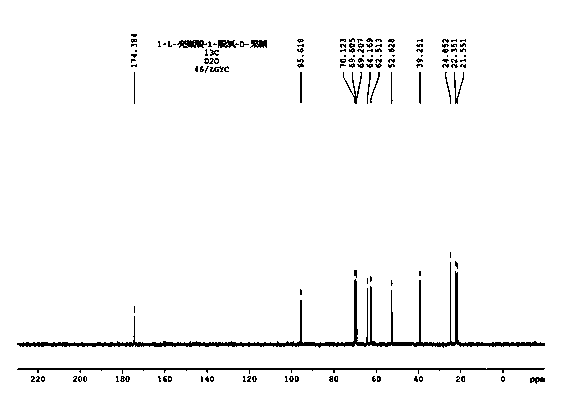
Cigarette aroma enhancement humectant 1-L-leucine-1-deoxidation-D-fructose and preparation method thereof
A technology of leucine and humectant, which is applied in the preparation of sugar derivatives, chemical instruments and methods, tobacco and other directions, can solve the problems of low temperature and easy solidification, no moisture-proof effect, and precipitation, etc., and achieves the effect of a reasonable synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation method of 1-L-leucine-1-deoxy-D-fructose includes the following steps:
[0025] Weigh 0.06 mol of D-mannose into a 500 mL three-necked flask, add 180 mL of anhydrous methanol, magnetically stir and reflux for 20 minutes, then add 0.06 mol of leucine and 0.0012 mol of citric acid in sequence, and then reflux again for 5 hours to complete the reaction. After the reaction solution is cooled to room temperature, unreacted D-mannose and amino acids are filtered out, and then concentrated under reduced pressure, separated by strong acid ion exchange resin column chromatography and recrystallized to obtain 8.795 g of the white target product with a yield of 40.03%.
[0026] During separation, the synthetic sample aqueous solution is added to the resin column, and then the unreacted D-mannose and uncharged substances are eluted with distilled water, and then washed with 1mol / L HCl aqueous solution, and the specific method is used at any time Monitor the eluent. When ...
Embodiment 2
[0028] The preparation method of 1-L-leucine-1-deoxy-D-fructose includes the following steps:
[0029] Weigh 0.06 mol of D-mannose into a 500 mL three-necked flask, add 240 mL of anhydrous methanol, magnetically stir and reflux for 40 min, then add 0.072 mol of leucine and 0.0024 mol of citric acid in sequence, and then reflux for another 7 hours to complete the reaction. After the reaction solution was cooled to room temperature, unreacted D-mannose and amino acids were filtered out, and then concentrated under reduced pressure, separated by strong acid ion exchange resin column chromatography and recrystallized to obtain 14.964 g of the white target product, with a yield of 68.11%.
[0030] During separation, the synthetic sample aqueous solution is added to the resin column, and then the unreacted D-mannose and uncharged substances are eluted with distilled water, and then washed with 1mol / L HCl aqueous solution, and the specific method is used at any time Monitor the eluent. Wh...
Embodiment 3
[0032] The preparation method of 1-L-leucine-1-deoxy-D-fructose includes the following steps:
[0033] Weigh 0.06 mol of D-mannose into a 500 mL three-necked flask, add 300 mL of anhydrous methanol, magnetically stir and reflux for 60 min, then add 0.084 mol of leucine and 0.003 mol of citric acid in sequence, and then reflux for another 9 hours to complete the reaction. After the reaction solution is cooled to room temperature, unreacted D-mannose and amino acids are filtered out, and then concentrated under reduced pressure, separated by strong acid ion exchange resin column chromatography and recrystallized to obtain 10.546 g of the white target product, with a yield of 48.00%.
[0034] In the separation, the synthetic sample aqueous solution is added to the resin column, and then the unreacted D-mannose and uncharged substances are eluted with distilled water, and then washed with 1M HCl aqueous solution, and the washing is monitored at any time by a specific method. Deliquorin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com