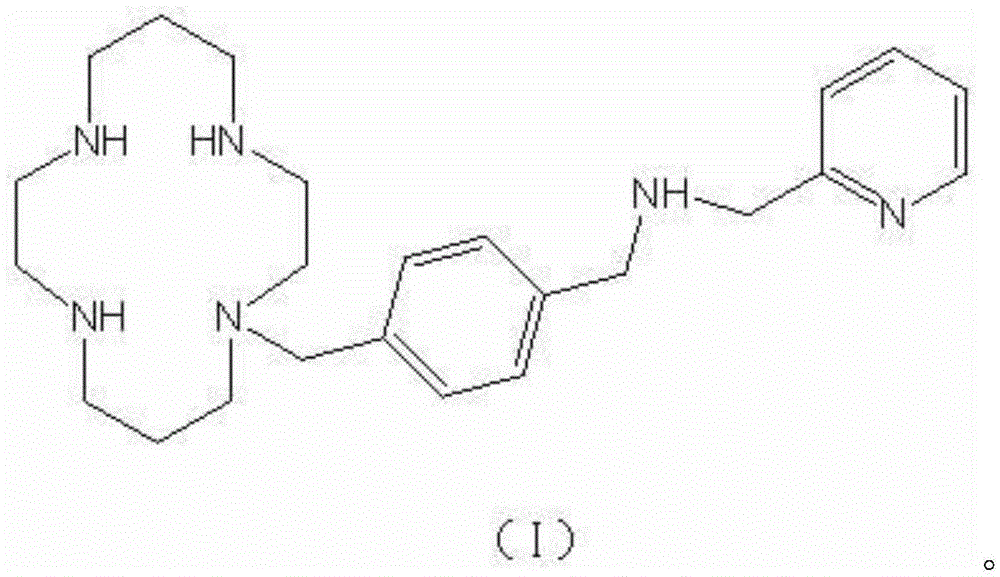
A kind of preparation technology of amd3465
A preparation process and disease technology, which is applied in the new process field of compound AMD3465 synthesis, can solve the problems of large-scale production operation difficulty, difficult source of tetraazacyclotetradecane, high price, etc., achieve good promotion and application prospects, and be easy to industrialize The effect of large production and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
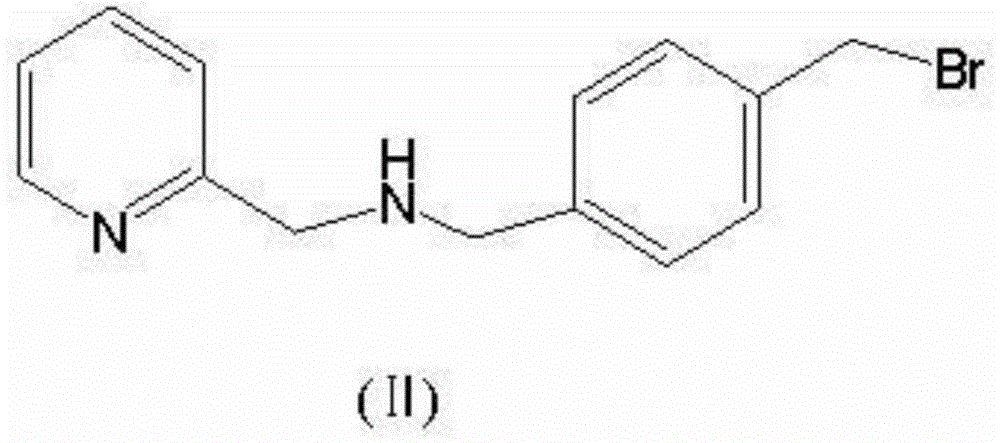
[0012] Add 109 grams of 2-aminomethylpyridine, 264 grams of p-dibromobenzyl, 200 grams of anhydrous potassium carbonate, and 1800 mL of acetonitrile into a reaction flask, heat to 70°C, react for 2 hours, cool to room temperature, and filter out potassium carbonate. The solvent was evaporated to dryness under reduced pressure, and the residue was poured into 1000 mL of water, stirred for 30 minutes, and filtered to obtain a light yellow solid. The solid was added to 1000 mL of methanol and stirred at room temperature for 1 hour, and filtered to obtain 252.3 g of solid (4-(bromomethyl)benzyl)-N-(pyridin-2-ylmethyl)methanamine, with a yield of 87.0%. ESI (M / Z) 291.2 (M+H+), elemental analysis (C14H15N2Br) measured value (theoretical value) C57.70% (57.75%), H5.20% (5.19%), N9.61% (9.62%) .
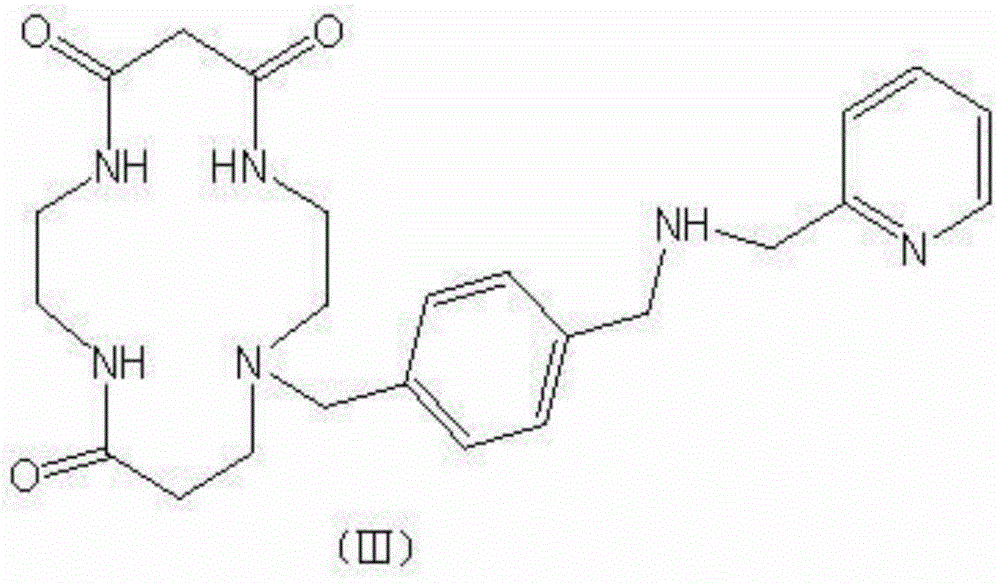
[0013] Dissolve 252 grams of (4-(bromomethyl)benzyl)-N-(pyridin-2-ylmethyl)methanamine, 210.0 grams of tricarbonyl ring ring, and 110 grams of anhydrous potassium carbonate in 1500 mL of ac...
Embodiment 2
[0016] Add 109 grams of 2-aminomethylpyridine, 264 grams of p-dibromobenzyl, 200 grams of anhydrous sodium carbonate, and 800 mL of DMF into a reaction flask, heat to 80°C, react for 2 hours, cool to room temperature, and filter out anhydrous sodium carbonate. The remainder of the reaction solution was poured into 2000 mL of ice water, stirred for 30 minutes, and filtered to obtain a light yellow solid. The solid was added to 1000 mL of methanol and stirred at room temperature for 1 hour, and filtered to obtain 239.3 g of solid (4-(bromomethyl)benzyl)-N-(pyridin-2-ylmethyl)methanamine, with a yield of 87.0%. ESI (M / Z) 291.2 (M+H+), elemental analysis (C14H15N2Br) measured value (theoretical value) C57.70% (57.75%), H5.20% (5.19%), N9.61% (9.62%) .
[0017] Dissolve 239 grams of (4-(bromomethyl)benzyl)-N-(pyridin-2-ylmethyl)methanamine, 220.0 grams of tricarbonyl ring ring, and 120 grams of anhydrous potassium carbonate in 1500 mL of acetonitrile, 80 After reacting at ℃ for 3...
Embodiment 3
[0020] Add 115 grams of 2-aminomethylpyridine, 302 grams of p-dibromobenzyl, 205 grams of anhydrous sodium carbonate, and 800 mL of DMF into a reaction flask, heat to 80°C, react for 2 hours, cool to room temperature, and filter out anhydrous sodium carbonate. The remainder of the reaction solution was poured into 2000 mL of ice water, stirred for 35 minutes, and filtered to obtain a light yellow solid. The solid was added to 1000 mL of methanol and stirred at room temperature for 1 hour, and filtered to obtain 241.5 g of solid (4-(bromomethyl)benzyl)-N-(pyridin-2-ylmethyl)methanamine, with a yield of 87.6%. ESI (M / Z) 291.2 (M+H+), elemental analysis (C14H15N2Br) measured value (theoretical value) C57.70% (57.75%), H5.20% (5.19%), N9.61% (9.62%) .
[0021] Dissolve 241.5 grams of (4-(bromomethyl)benzyl)-N-(pyridin-2-ylmethyl)methanamine, 231.0 grams of tricarbonyl ring ring, and 116 grams of anhydrous sodium carbonate in 1500 mL of acetonitrile, 75 After reacting at ℃ for 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com