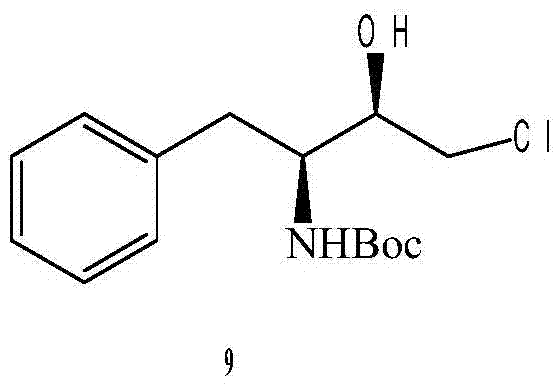
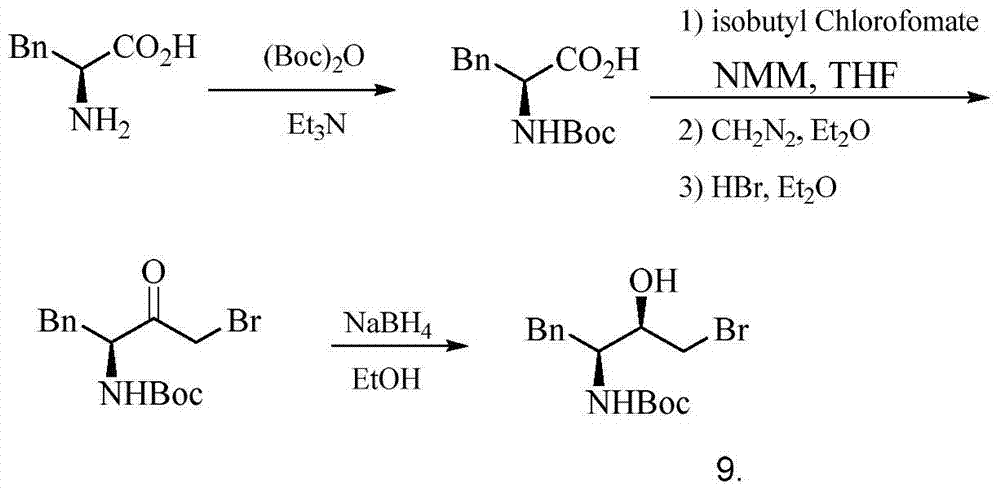
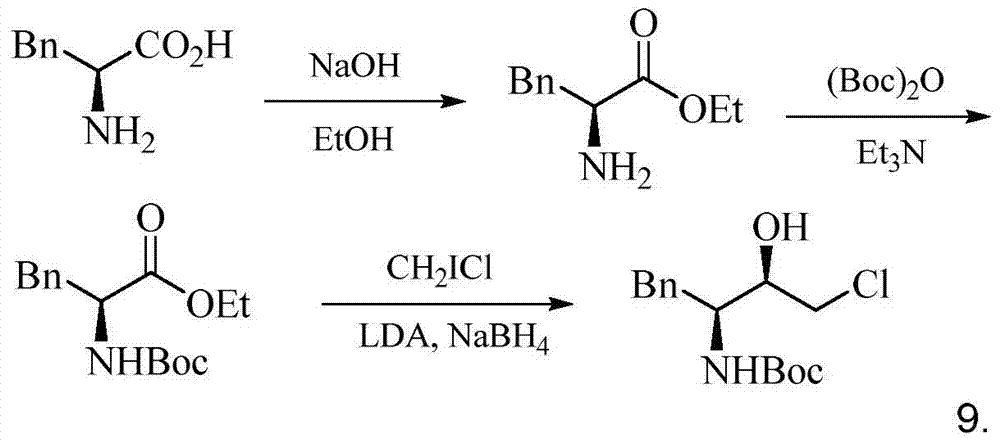
A kind of preparation method of (2r,3s)-1-chloro-3-tert-butoxyamido-4-phenyl-2-butanol
A kind of tert-butoxyamido, phenyl technology, applied in the preparation of -1-chloro-3-tert-butoxyamido-4-phenyl-2-butanol, the field of anti-AIDS drug amprenavir intermediates , can solve the problems of unsuitability for industrial production, difficult separation of products, harsh reaction conditions, etc., to achieve safe yield, improve production efficiency, and achieve the effect of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Step 1: (S)-2-(Dibenzylamino)-3-phenyl-propionic acid benzyl ester 2
[0040] L-phenylalanine 1 (20.0g, 121.1mmol), K 2 CO 3 (60.0g, 434.8mmol), H 2 The mixture of O (90ml), EtOH (45ml), and BnCl (45.2g, 394.7mmol) was heated to 90°C, and the reaction was stopped after 15h. After the reaction, the aqueous layer was removed, 100ml of n-hexane was added to the organic layer and washed with 500ml of water, dried, filtered, and spin-dried to obtain light yellow liquid compound 2 (50.6g, 96%). 1 H-NMR (300MHz, CDCl 3 ):δ3.20(dd,2H,J=8.4,14.4Hz,Ph CH 2 C),3.60(d,2H,J=15.0Hz,2Ph CHb N),3.80(dd,1H,J=8.5,8.5Hz,N CH ),4.00(d,2H,J=15.0Hz,2PhCHb N),5.20(d,1H,J=13.5Hz,Ph CHb O),5.30(d,1H,J=13.5Hz,Ph CHb O),7.50-7.00(m,20H,4PhH)ppm. 13 C-NMR (125MHz, CDCl 3 ):δ35.6,54.3,62.3,66.0,126.2,126.9,128.1,128.2,128.4,128.5,128.6,129.4,135.9,138.0,139.2,172.0ppm.MS(ESI,m / z):436.0(MH + ).
[0041] Step 2: N,N-Dibenzyl-L-phenylalanine 3
[0042] (S)-2-(Dibenzylamino)-3-phenyl-pr...
Embodiment 2
[0056] Step 1: (S)-2-(Dibenzylamino)-3-phenyl-propionic acid benzyl ester 2
[0057] Dissolve L-phenylalanine 1 (50.0g, 302.7mmol), NaOH (20.0g, 500.0mmol), BnCl (294.9g, 908.7mmol) in 250ml of water and 200ml of EtOH, heat to reflux at 90°C, N 2 Reacted at this temperature for 10 h under protection. After the reaction was completed, toluene (2×250ml) was added, and several layers were washed with water and saturated brine in sequence, dried, filtered, and spin-dried to obtain light yellow liquid compound 2 (121.3g, 92 %).
[0058] The second step: (S)-N,N-dibenzyl-L-phenylalanine 3
[0059] With embodiment 1.
[0060] Step 3: Bn-mixed anhydride 4
[0061] A 500ml glass reactor was equipped with a 250ml addition funnel, stirrer, thermometer and cooling bath. Dissolve (S)-N,N-dibenzyl-L-phenylalanine 3 (50.0g, 130.9mmol) in dichloromethane (200ml), and add N-methylmorpholine at one time while stirring (16.0 g), the clear, colorless solution was transferred to an addition f...
Embodiment 3
[0073] Step 1: (S)-2-(Dibenzylamino)-3-phenyl-propionic acid benzyl ester 2
[0074] L-phenylalanine 1 (20.0g, 122.7mmol), K 2 CO3 (60.0g, 606mmol), H 2 The mixture of O (90ml), EtOH (45ml), and BnCl (50.0g, 393.7mmol) was heated to 80°C, and the reaction was stopped after 48h. After the reaction, the aqueous layer was removed, 100ml of n-hexane was added to the organic layer and washed with 500ml of water, dried, filtered, and spin-dried to obtain light yellow liquid compound 2 (51.7g, 96.8%).
[0075] The second step: (S)-N,N-dibenzyl-L-phenylalanine 3
[0076] With embodiment 1.
[0077] Step 3: Bn-mixed anhydride 4
[0078] A 250ml glass reactor was equipped with a 250ml addition funnel, stirrer, thermometer and cooling bath. Dissolve (S)-N,N-dibenzyl-L-phenylalanine 3 (25.0g, 0.065mol) in dichloromethane (100ml), and add N-methylmorpholine in one go while stirring (8.78 g), the clear, colorless solution was transferred to an addition funnel. A solution of ethyl chl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com