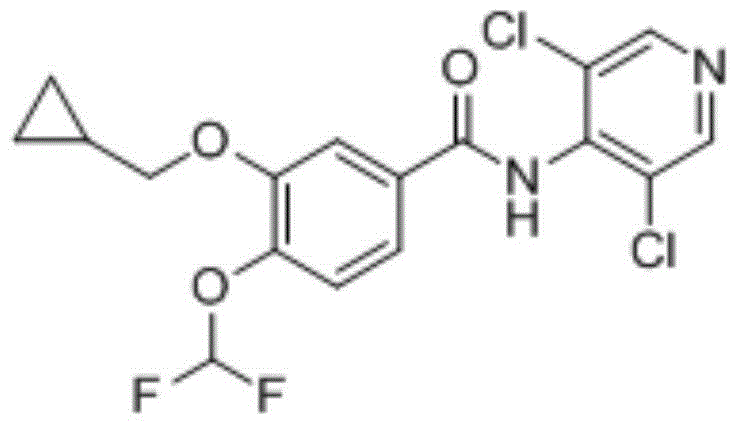
Roflumilast dispersible tablet composition and preparation method thereof
A technology for roflumilast and dispersible tablets is applied in the field of roflumilast dispersible tablet composition and preparation thereof, and can solve the problems of strong hygroscopicity, influence on dissolution rate, low bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
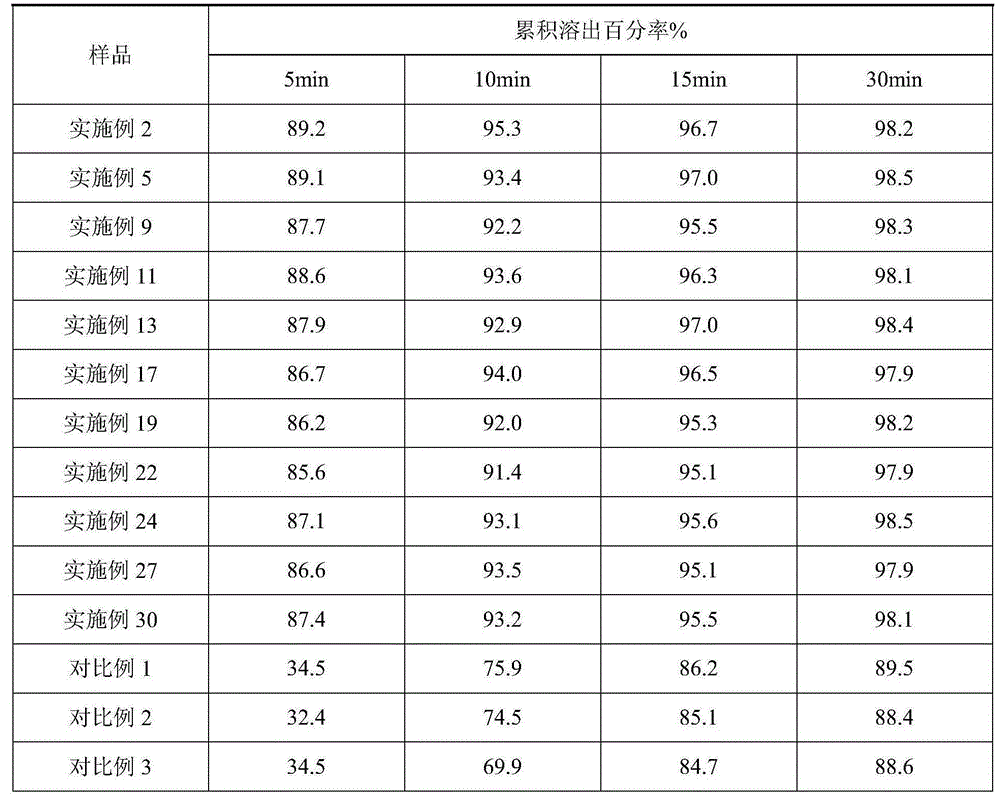
Examples
Embodiment 1~3
[0062] Examples 1-3: (based on 1000 pieces, unit: g)
[0063] Raw materials
Embodiment 1
[0064] Embodiment 1 preparation method:
[0065] Co-micronize the prescribed amount of roflumilast and mannitol at a mass ratio of 1:10, sieve to obtain the micronized material, and set aside; prepare the prescribed amount of copovidone into a 10% (w / w) aqueous solution, Standby; add the remaining amount of mannitol, micronized materials, lactose and crospovidone into the mixing hopper in sequence and mix to obtain the premixed powder of roflumilast dispersible tablets; premix the roflumilast dispersible tablets The powder is placed in a granulator, and copovidone aqueous solution is added to make wet granules; the wet granules are dried; the prescribed amount of sodium stearyl fumarate is added, and mixed; compressed into tablets, and the product is obtained.
Embodiment 2
[0066] Embodiment 2 preparation method is the same as embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com