Valsartan/hydrochlorothiazide pharmaceutical composition and preparation method thereof
A technology of hydrochlorothiazide and composition is applied in the field of pharmaceutical composition containing valsartan and hydrochlorothiazide and its preparation field, which can solve the problems of unsatisfactory, large difference in tablet weight, drug disintegration and dissolution, etc. Fast, fluid results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
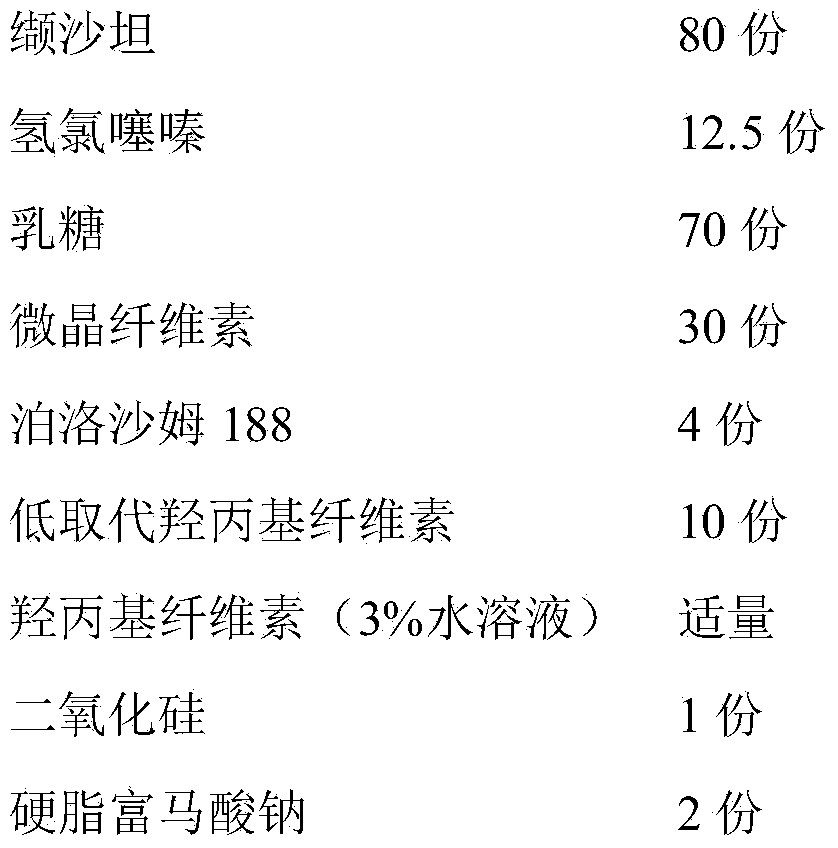
[0049] 1. Prescription
[0050]
[0051] 2. Preparation process
[0052] 1) Carry out micronization treatment with valsartan and hydrochlorothiazide, control particle size to be no more than 50 microns, take by prescription quantity, standby;
[0053] 2) Prepare 3% hydroxypropyl cellulose aqueous solution, and take a small part to add the prescribed amount of poloxamer, stir to dissolve;
[0054] 3) Weighing valsartan, hydrochlorothiazide, lactose, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose according to the prescription amount, and mixing them uniformly to obtain a mixed powder;
[0055] 4) Slowly add the above-mentioned 3% hydroxypropyl cellulose aqueous solution containing poloxamer to the mixed powder, mix well, then add an appropriate amount of 3% hydroxypropyl cellulose aqueous solution to make soft material, granulate with a 20-mesh sieve, Dry at 80°C, and sieve with 18 meshes to obtain granules;
[0056] 5) The prescription amount of sil...
Embodiment 2
[0058] 1. Prescription
[0059]
[0060] 2. Preparation process
[0061] 1) Carry out micronization treatment with valsartan and hydrochlorothiazide, control particle size to be no more than 50 microns, take by prescription quantity, standby;
[0062] 2) Prepare 3% hydroxypropyl cellulose aqueous solution, and take a small part to add the prescribed amount of poloxamer, stir to dissolve;
[0063] 3) Weighing valsartan, hydrochlorothiazide, lactose, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose according to the prescription amount, and mixing them uniformly to obtain a mixed powder;
[0064] 4) Slowly add the above-mentioned 3% hydroxypropyl cellulose aqueous solution containing poloxamer to the mixed powder, mix evenly, then add an appropriate amount of 3% hydroxypropyl cellulose aqueous solution to make soft material, granulate with a 40-mesh sieve, Dry at 80°C, and sieve with 40 mesh to obtain granules;
[0065] 5) The prescription amount of s...
Embodiment 3
[0067] 1. Prescription
[0068]
[0069] 2. Preparation process
[0070] 1) Carry out micronization treatment with valsartan and hydrochlorothiazide, control particle size to be no more than 50 microns, take by prescription quantity, standby;
[0071] 2) Prepare 3% hydroxypropyl cellulose aqueous solution, and take a small part to add the prescribed amount of poloxamer, stir to dissolve;
[0072] 3) Weighing valsartan, hydrochlorothiazide, lactose, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose according to the prescription amount, and mixing them uniformly to obtain a mixed powder;
[0073] 4) Slowly add the above-mentioned 3% hydroxypropyl cellulose aqueous solution containing poloxamer to the mixed powder, mix well, then add an appropriate amount of 3% hydroxypropyl cellulose aqueous solution to make a soft material, granulate with a 24-mesh sieve, Dry at 70°C, and sieve through a 20-mesh sieve to obtain granules;
[0074] 5) The prescription ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com