Rebeprazole sodium plain tablets, rebeprazole sodium enteric tablets and preparation method of rebeprazole sodium enteric tablets
A technology of rabeprazole sodium tablets and rabeprazole sodium, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of bioavailability of rabeprazole sodium Low rate, poor dissolution of tablet cores and other problems, to achieve the effect of high hardness, high dissolution and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
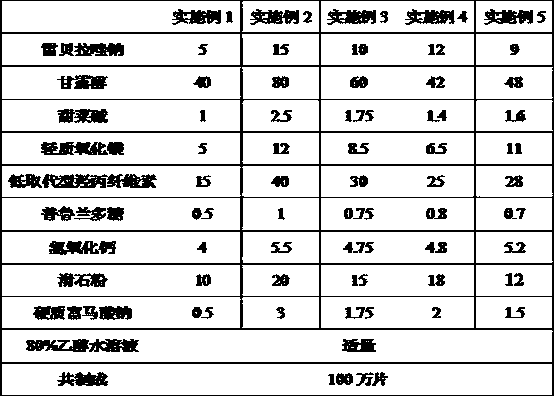
[0056] A kind of rabeprazole sodium element tablet, comprises the component of following weight portion:
[0057] Rabeprazole sodium 5-15 parts;
[0058] Mannitol 40-80 parts;
[0059] Betaine 1-2.5 parts;
[0060] 5-12 parts of light magnesium oxide;
[0061] Low-substituted hydroxypropyl cellulose 15-40 parts;
[0062] 0.5-1 part of pullulan polysaccharide;
[0063] 4-5.5 parts of calcium hydroxide;
[0064] 10-20 parts of talcum powder;
[0065] 0.5-3 parts of sodium stearyl fumarate.
[0066] Preferably, the granules of rabeprazole sodium, mannitol, betaine, light magnesium oxide, low-substituted hydroxypropyl cellulose, pullulan, calcium hydroxide, talc and hard sodium fumarate The diameter is 60-90 mesh; more preferably 80 mesh.
[0067] In some preferred embodiments, the mass ratio of mannitol to betaine is 30:1.
[0068] In some preferred embodiments, the rabeprazole sodium tablet also includes 4-5.5 parts by weight of meglumine, and more preferably, the mass ...
experiment example 1
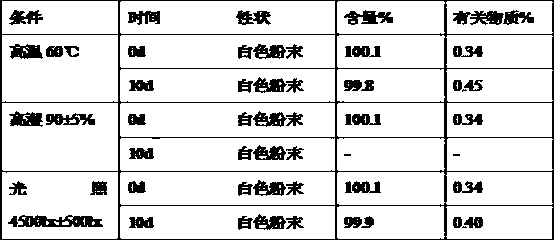
[0083] Experimental example 1. Compatibility of raw materials and auxiliary materials.
[0084] Rabeprazole sodium is mixed with betaine and meglumine respectively in a mass ratio of 1:5, and rabeprazole sodium and pullulan are mixed in a mass ratio of 15:1, according to the 2010 edition of "Chinese Pharmacopoeia" Part Two Appendix XIXC-Guiding Principles for Stability Testing of APIs and Pharmaceutical Preparations Carry out testing of influencing factors to test the properties, related substances and content of the mixture. The test results are shown in Table 3-6.
[0085] Table 3 Rabeprazole sodium raw material experimental results
[0086]
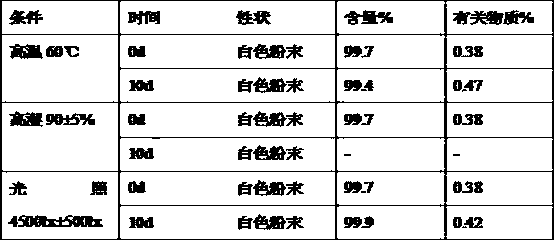
[0087] The compatibility test result of table 4 rabeprazole sodium and betaine
[0088]
[0089] The compatibility test result of table 5 rabeprazole sodium and meglumine
[0090]
[0091] Table 6 Compatibility test results of rabeprazole sodium and pullulan
[0092]
[0093] It can be seen from Table 3-6 that after pla...
experiment example 2
[0094] Experimental Example 2 Dissolution of Rabeprazole Sodium Tablets
[0095] Get Rabeprazole Sodium Tablets, adopt dissolution assay (Chinese Pharmacopoeia 2010 edition two appendix XC first method) device, with phosphate buffer (pH6.8) (get 0.1mol / L hydrochloric acid solution and 0.2mol / L sodium phosphate solution, mix uniformly according to 3:1, if necessary, use 2mol / L hydrochloric acid solution or 2mol / L sodium hydroxide solution to adjust the pH value to 6.8) 1000mL is used as the solvent, and the speed is 75 revolutions per minute. Operate according to the law. At 45 minutes, take 10 mL of the solution, filter it, take the subsequent filtrate, add phosphate buffer (pH6.8) to dilute to make a solution containing about 10 μg per 1 mL, shake well, and use it as the test solution; Sodium prazole reference substance 10mg, accurately weighed, put in a 100mL measuring bottle, dissolve with water and dilute to the mark, shake up, accurately measure 5mL, put in a 50mL measur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com