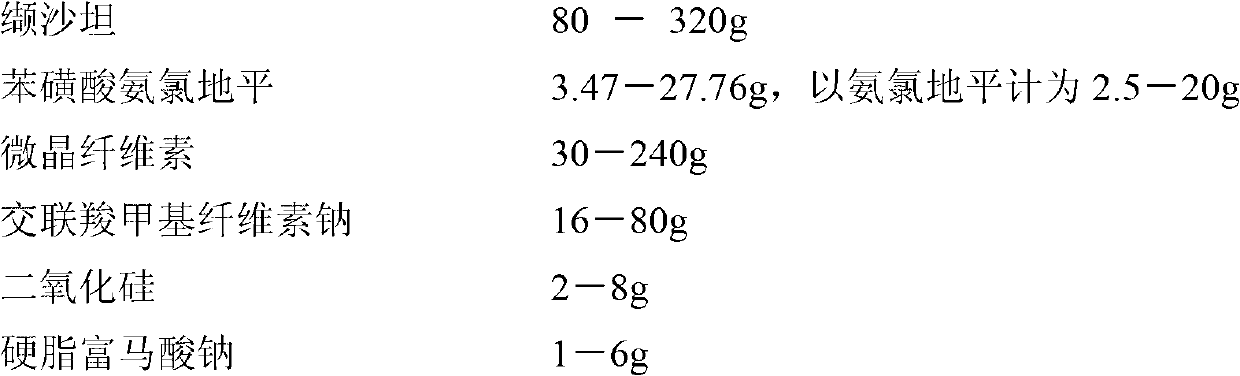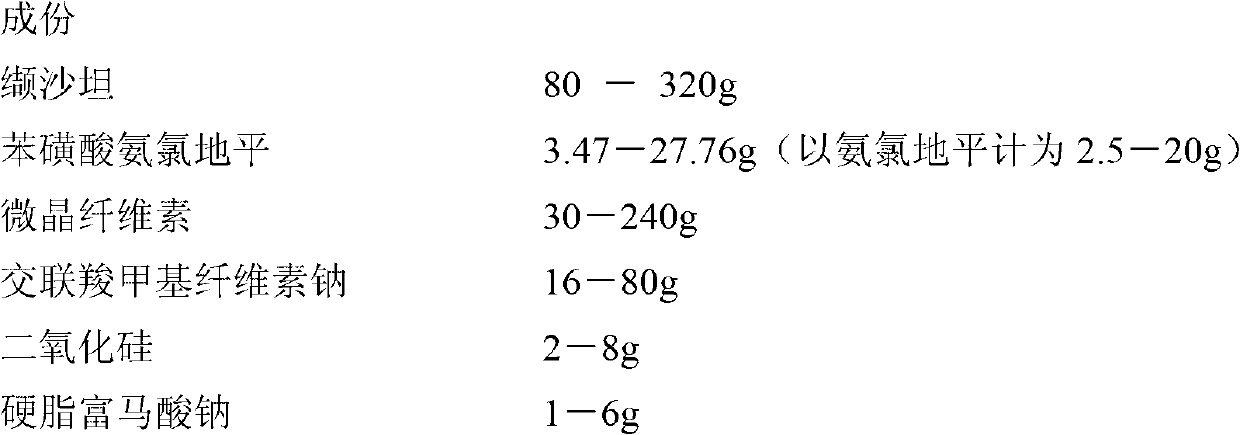Pharmaceutical composition containing valsartan and amlodipine besylate and preparation method
A kind of technology of valsartan amlodipine besylate and composition, which is applied to the pharmaceutical composition containing valsartan amlodipine besylate and the field of preparation thereof, and can solve the problem of unsatisfactory blood pressure, patients failing to reach the target target, etc. problems, to achieve the effects of stable quality, good adhesion and strict control in accelerated and long-term tests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067]
[0068]
[0069] Its preparation process is:
[0070] (1) Pretreatment: Take raw materials and auxiliary materials, pass through 60-mesh sieve respectively, and set aside.
[0071] (2) Dry mixing: mix amlodipine besylate with 1 / 2 of croscarmellose sodium, then mix with microcrystalline cellulose, silicon dioxide and valsartan, and mix evenly.
[0072] (3) Granulation: dry granulation, 18 mesh granulation.
[0073] (4) Blending: Mix the dry granules, the remaining half of the croscarmellose sodium and sodium stearyl fumarate evenly. Measure the content of intermediates.
[0074] (5) Tablets. According to the results of the determination of the intermediates, the weight of the tablet is calculated, and the tablet is pressed.
[0075] (6) Film coating. The film coating premix is used to prepare a 10% coating solution, the temperature is controlled to be 30-40° C., and the coating is carried out, and the weight of the coating is increased by 3%, and then dried...
Embodiment 2
[0078]
[0079] Its preparation process is:
[0080] (8) Pretreatment: Take raw materials and auxiliary materials, pass through 60-mesh sieve respectively, and set aside.
[0081] (9) Dry blending: mix amlodipine besylate with half of croscarmellose sodium, and then mix with microcrystalline cellulose, silicon dioxide and valsartan, and mix evenly.
[0082] (10) Granulation: dry granulation, 18 mesh granulation.
[0083] (11) Total blending: Mix the dry granules, the remaining half of the croscarmellose sodium, and sodium stearyl fumarate evenly. Measure the content of intermediates.
[0084] (12) Tablets. According to the results of the determination of the intermediates, the weight of the tablet is calculated, and the tablet is pressed.
[0085] (13) Film coating. The film coating premix is used to prepare a 10% coating solution, the temperature is controlled to be 30-40° C., and the coating is carried out, and the weight of the coating is increased by 3%, and then...
Embodiment 3
[0088]
[0089] Its preparation process is:
[0090] (15) Pretreatment: Take raw materials and auxiliary materials, pass through 60-mesh sieve respectively, and set aside.
[0091] (16) Dry mixing: mix amlodipine besylate with half of croscarmellose sodium, then mix with microcrystalline cellulose, silicon dioxide and valsartan, and mix evenly.
[0092] (17) Granulation: dry granulation, 18 mesh granulation.
[0093] (18) Total blending: Mix the dry granules, the remaining half of the croscarmellose sodium, and sodium stearyl fumarate evenly. Measure the content of intermediates.
[0094] (19) Tablets. According to the results of the determination of the intermediates, the weight of the tablet is calculated, and the tablet is pressed.
[0095] (20) Film-coated. The film coating premix is used to prepare a 10% coating solution, the temperature is controlled to be 30-40° C., and the coating is carried out, and the weight of the coating is increased by 3%, and then drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com