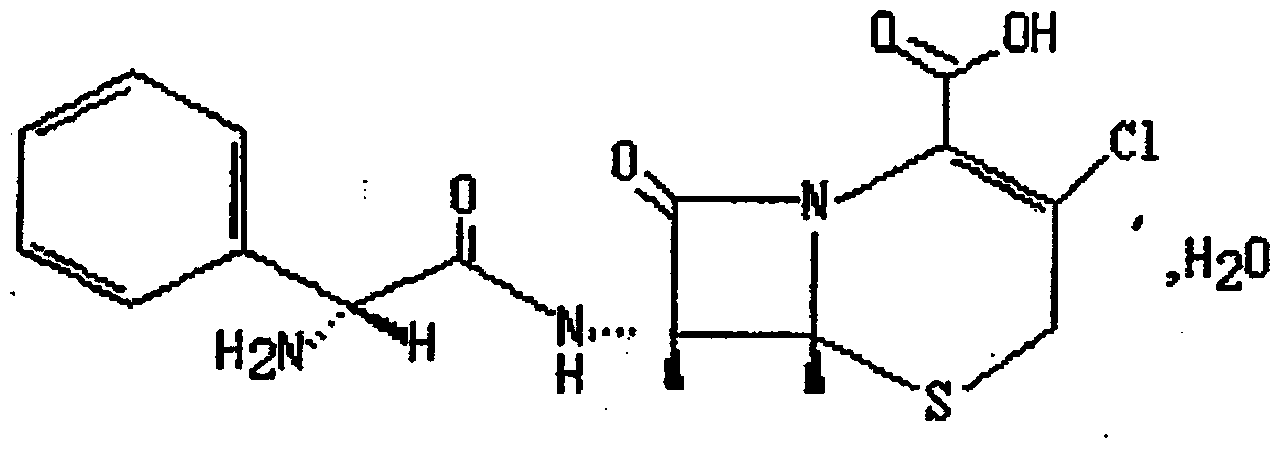
Stable cefaclor tablet composition and preparation method thereof
A technology for cefaclor and a composition, which is applied in the field of pharmaceutical compositions and their preparation, can solve the problems of decreased dissolution of cefaclor tablets, increased related substances, decreased content, etc., so as to improve solubility and bioavailability, and ensure product quality quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 cefaclor tablet prescription:
[0032]
[0033]
[0034] The preparation process is as follows:
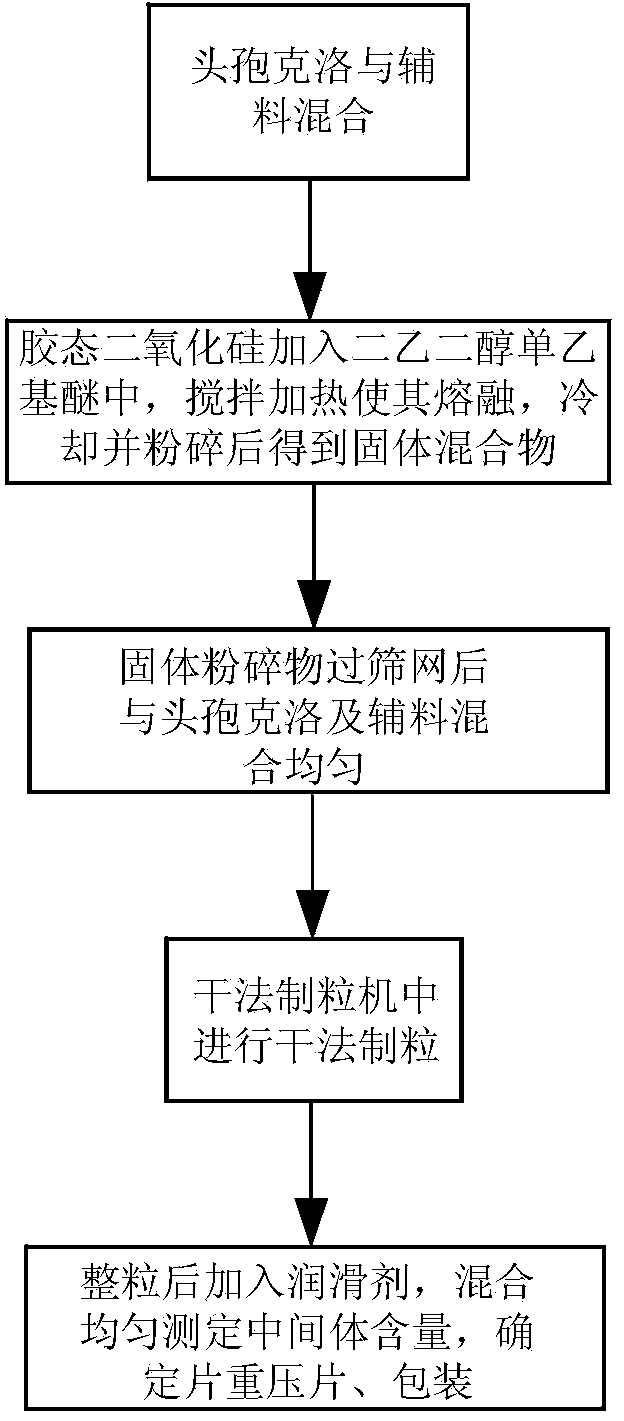
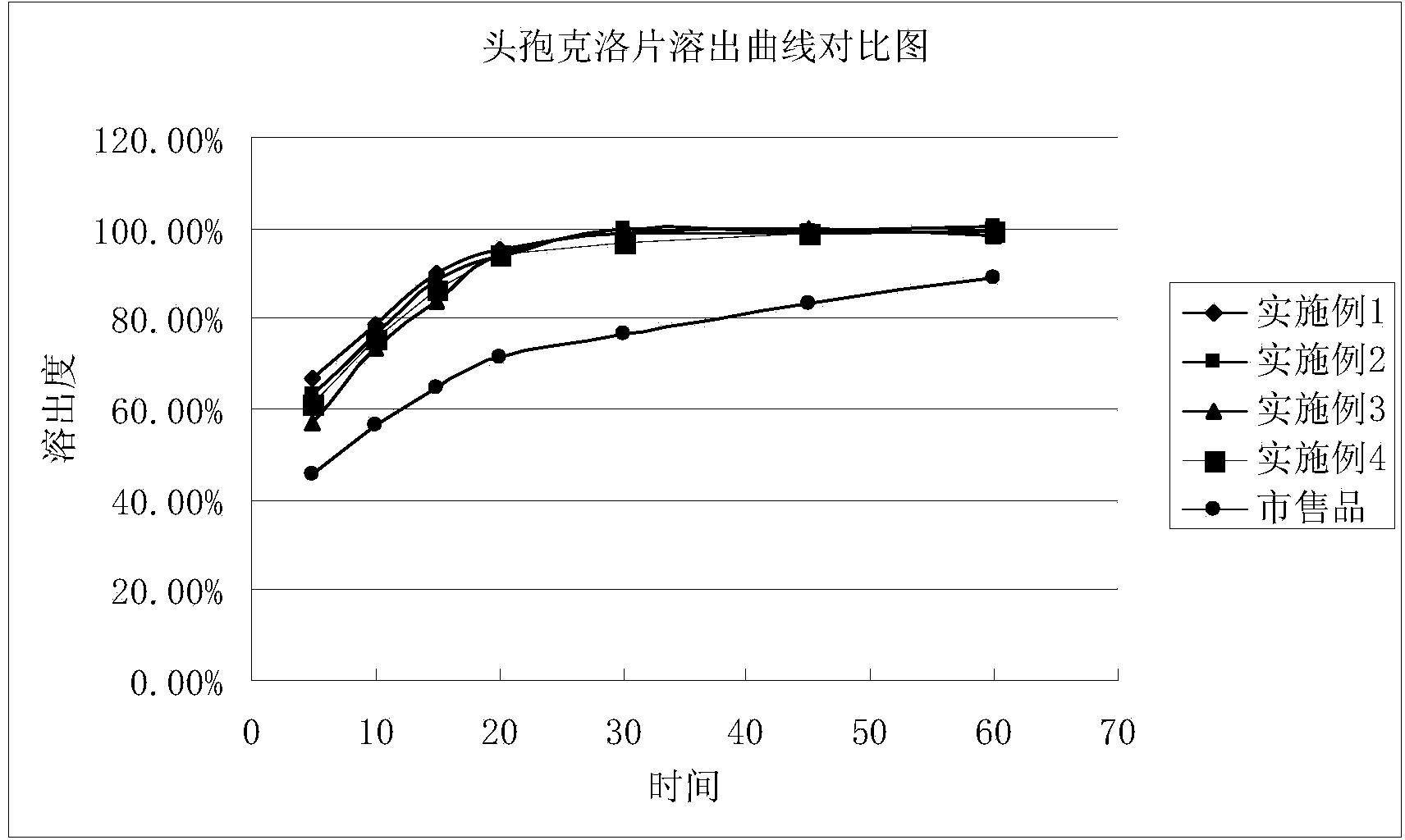
[0035] Such as figure 1 As shown, the cefaclor, microcrystalline cellulose, lactose, and crospovidone were mixed uniformly, the colloidal silicon dioxide was added to diethylene glycol monoethyl ether and stirred evenly while heating, Make it melt, cool and pulverize, pass through a 60-mesh sieve, add cefaclor and prescribed amounts of microcrystalline cellulose, lactose, and crospovidone into a tank mixer, stir for 20 minutes, and dry the mixture The granulator granulates to obtain dry granules, and passes the dry granules through a 16-mesh sieve for granulation. Mix the granules obtained after granulation with the prescribed amount of glyceryl behenate in a V-type mixer for 10 minutes, measure the content of intermediates, determine the weight of the tablets, press them into tablets, and pack the plain tablets to obtain the product.
Embodiment 2
[0036] Embodiment 2 cefaclor tablet prescription:
[0037]
[0038] The preparation process is as follows:
[0039] Such as figure 1 As shown, the cefaclor, microcrystalline cellulose, lactose, and crospovidone are mixed uniformly, colloidal silicon dioxide is added to diethylene glycol monoethyl ether and stirred evenly while heating to make it Melt, cool and pulverize, pass through a 60-mesh sieve, add cefaclor and prescribed amounts of microcrystalline cellulose, lactose, and crospovidone into a tank mixer, stir for 20 minutes, and use a dry granulation mechanism for the mixture Granulate to obtain dry granules, and pass the dry granules through a 16-mesh sieve for granulation. Mix the granules obtained after granulation with the prescribed amount of glyceryl behenate in an elevating mixer for 10 minutes, measure the content of intermediates, determine the weight of the tablets, press them into tablets, and pack the plain tablets to obtain the product.
Embodiment 3
[0040] Embodiment 3 cefaclor tablet prescription:
[0041]
[0042] The preparation process is as follows:
[0043] Such as figure 1 As shown, the cefaclor, microcrystalline cellulose, lactose, and crospovidone are mixed uniformly, colloidal silicon dioxide is added to diethylene glycol monoethyl ether and stirred evenly while heating to make it Melt, cool and pulverize, pass through a 60-mesh sieve, add cefaclor and prescribed amounts of microcrystalline cellulose, lactose, and crospovidone into a tank mixer, stir for 20 minutes, and use a dry granulation mechanism for the mixture Granulate to obtain dry granules, and pass the dry granules through a 16-mesh sieve for granulation. Mix the granules obtained after granulation with the prescribed amount of sodium stearyl fumarate in a V-type mixer for 10 minutes, measure the content of intermediates, determine the weight of the tablet, compress it, and pack the plain tablet to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com