Pharmaceutical composition of enalapril maleate folic acid and preparation method thereof
A technology of enalapril maleate and prifolic acid, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve problems such as economic loss, decomposition and deterioration, and increase in side effects, so as to ensure safety Use, good stability, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
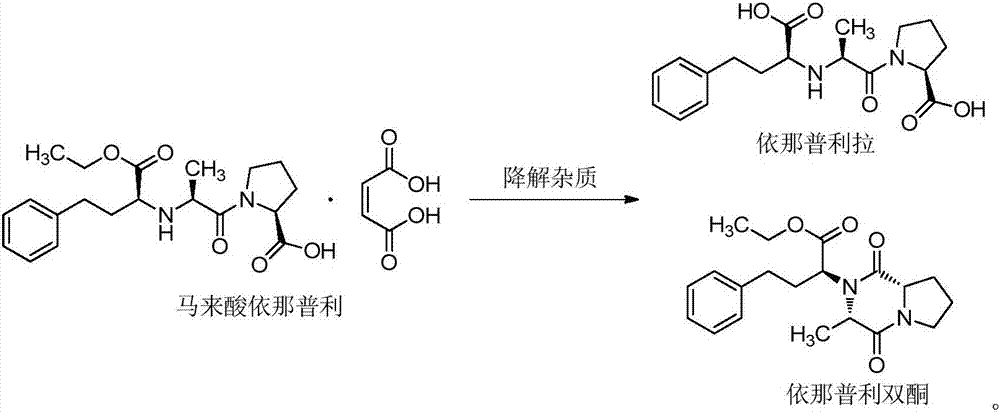
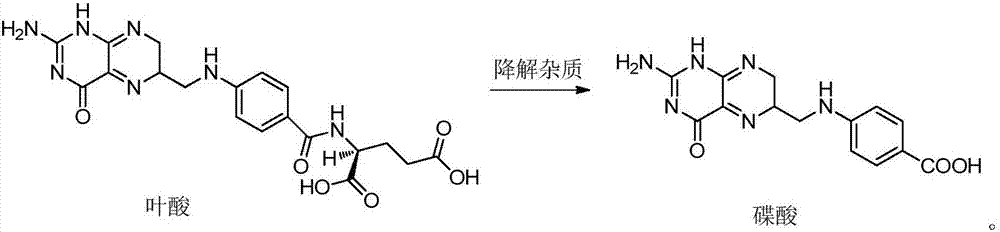
[0037] Example 1 Stability of raw materials
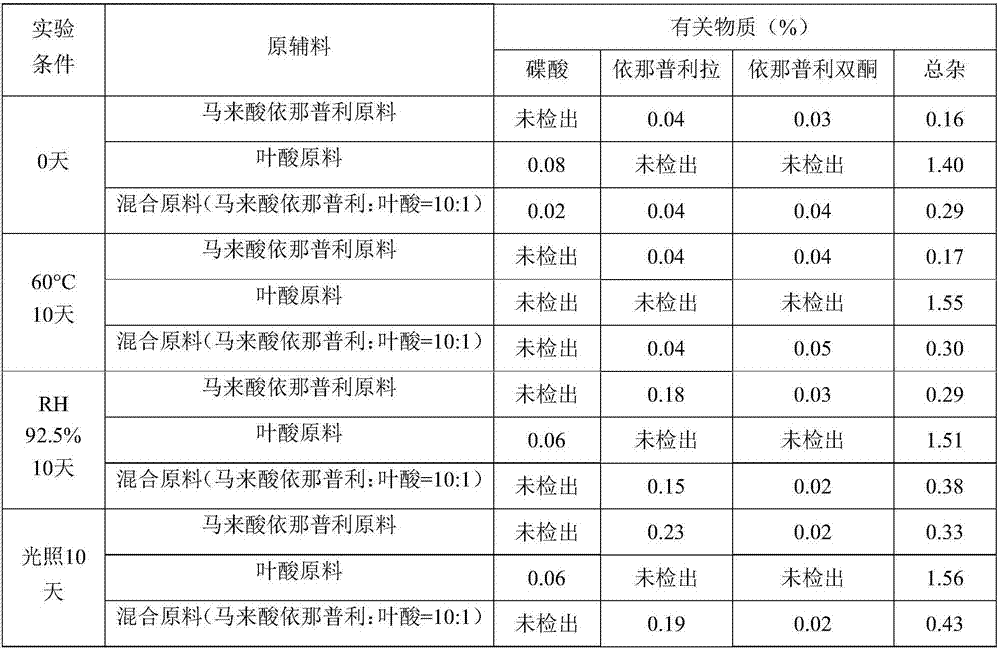
[0038] Put the mixed raw materials of enalapril maleate, folic acid, enalapril maleate and folic acid with a weight ratio of 10:1 under the conditions of 60℃ high temperature, humidity RH 92.5% and 4500lx strong light for 10 days. Influencing factor experiments, the results are shown in Table 1.
[0039] Related substance detection method: Waters high performance liquid chromatography e2695-2489 instrument detection, flow rate 2.0mL no detection min, column temperature: 50℃, detection wavelength 215nm, mobile phase A: 0.01mol L potassium dihydrogen phosphate buffer solution is not detected Adjust pH to 2.2, mobile phase B: acetonitrile, gradient elution mobile phase A did not detect the volume ratio of mobile phase B 90:10→70:30→90:10, only the main impurity content is listed in Table 1.
[0040] Table 1 Investigation of the stability of raw materials
[0041]
[0042] It can be seen from Table 1 that enalapril maleate, folic acid and the...
Embodiment 2
[0043] Example 2 Filler screening
[0044] Through the compatibility experiment of starch, lactose monohydrate, lactose anhydrous, microcrystalline cellulose 101, microcrystalline cellulose 102, mannitol and pregelatinized starch, the fillers were screened at high temperature 60℃ and high humidity. Placed for 10 days under RH 92.5% and 4500lx strong light irradiation conditions, the results are shown in Table 2.
[0045] Table 2 Compatibility experiment of filler raw materials
[0046]
[0047]
[0048] It can be seen from Table 2 that when starch, lactose monohydrate, anhydrous lactose, microcrystalline cellulose 101, microcrystalline cellulose 102, mannitol or pregelatinized starch and enalapril maleate folic acid raw materials are in high humidity When placed under RH 92.5% and light conditions for 10 days, there is no significant change in the impurities of dish acid, enalaprilat and enalapridone; but starch, microcrystalline cellulose 101, microcrystalline cellulose 102 or preg...
Embodiment 3
[0049] Example 3 Adhesive Screening
[0050] Through the original and adjuvant compatibility test of povidone K30, hypromellose E5, hydroxypropyl cellulose SL-FP and hypromellose 603, the adhesives were selected at high temperature of 60℃ and high humidity. Placed for 10 days under RH 92.5% and 4500lx strong light irradiation conditions, the results are shown in Table 3.
[0051] Table 3 Compatibility experiment of adhesive raw materials
[0052]
[0053] It can be seen from Table 3 that when povidone K30, hypromellose E5, hydroxypropyl cellulose SL-FP or hypromellose 603 and enalapril maleate folic acid raw materials are at a high temperature of 60℃, When placed under high humidity RH 92.5% and 4500lx light for 10 days, there is no significant change in impurity of betelic acid, enalaprilat and enalapridone, indicating that povidone K30, hypromellose E5, hydroxypropyl Cellulose SL-FP or hypromellose 603 has good compatibility with enalapril maleate folic acid raw materials, and ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com