Propafenone hydrochloride sustained-release capsule and preparation method thereof
A technology of propafenone hydrochloride and sustained-release capsules, which can be used in pharmaceutical formulations, medical preparations of non-active ingredients, cardiovascular system diseases, etc., and can solve problems such as large tablet volume, poor drug homogeneity, and low bioavailability , achieve the effect of stable curative effect, long-lasting drug effect and improved compliance
Inactive Publication Date: 2014-07-09
LP PHARM (XIAMEN) CO LTD
View PDF3 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0005] In 2003, the Propafenone Hydrochloride Sustained-release Capsules (12-hour sustained release) developed and produced by Abbott Laboratories of the U.S. went on the market. It is a kind of 2×2mm micro-tablets packed into capsules, and each capsule contains about 50-60 micro-tablets. The technical solution is disclosed in the PCT patent Chinese document CN1120312A, which is characterized in that propafenone hydrochloride and its auxiliary substances are prepared into micro-tablets. Sustained release of propafenone, its preparation process is complicated, and there are many types of excipients, which is not conducive to human body absorption and metabolism, and the clinical safety of the drug needs to be improved
[0011] The above domestic propafenone hydrochloride sustained-release preparations prepared by coating / skeleton and other sustained-release technologies have achieved sustained-release effects to a certain extent, but there are disadvantages: (1) The tablet volume is relatively large and the bioavailability is low At the same time, it is not popular with patients; (2) The homogeneity of the drug is relatively poor, and the coating effect is very high. Once there is a slight damage to the coating, the content of all active components will be released suddenly, resulting in side effects; (3) ) The bioefficiency of sustained-release pellets is greatly affected by food intake, with large inter-individual and intra-individual variability
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0041]
Embodiment 2
[0043]
[0044]
Embodiment 3
[0046]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Login to View More
Abstract
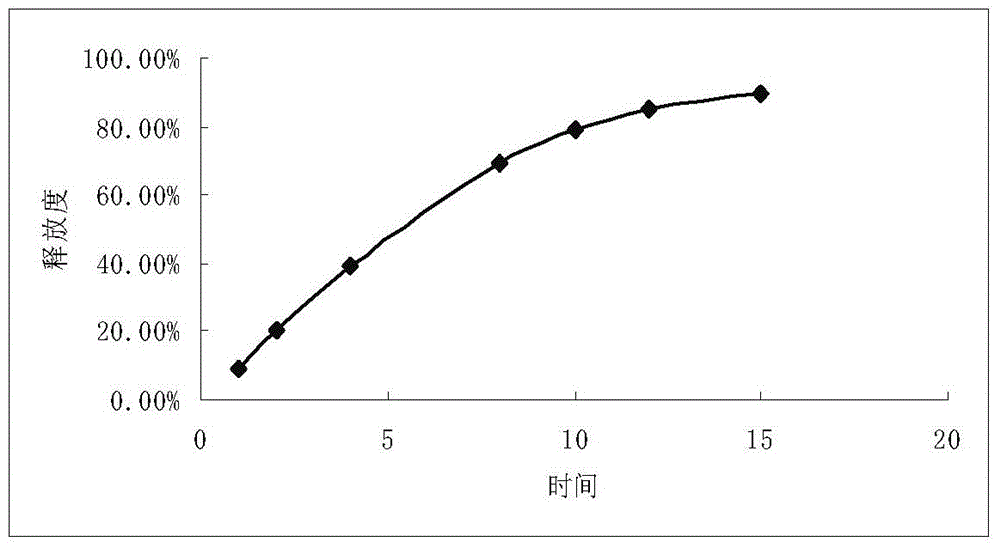
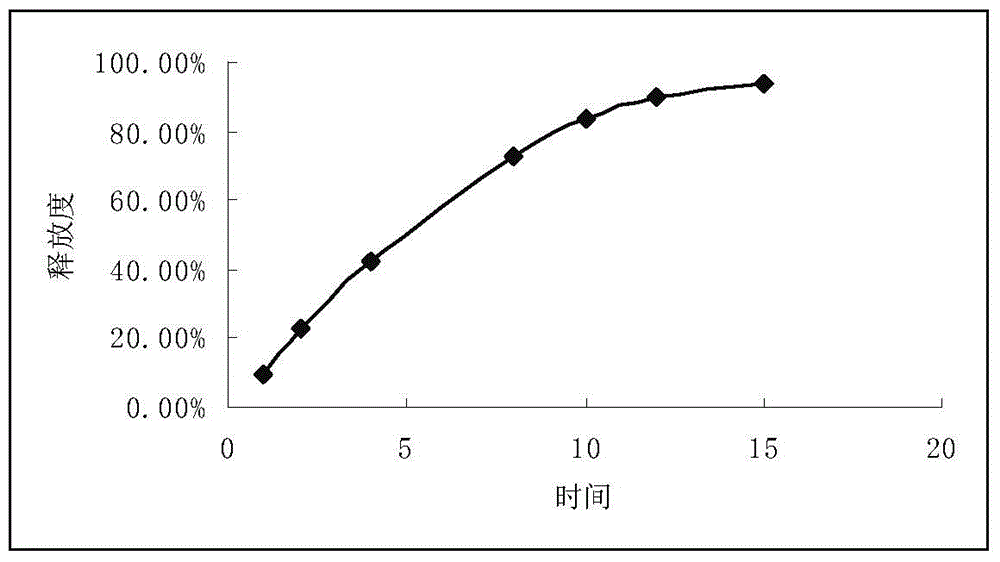
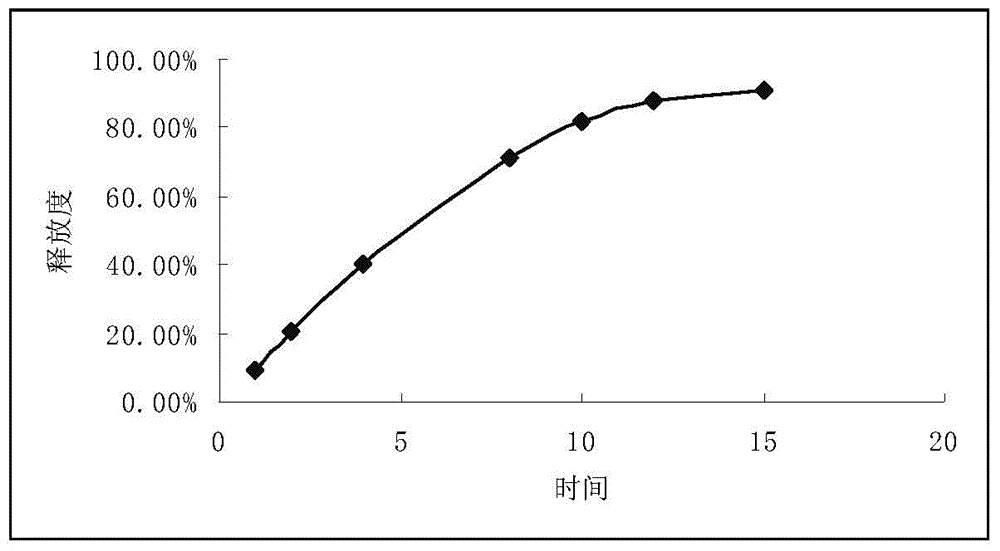
The invention relates to a propafenone hydrochloride sustained-release capsule and a preparation method thereof. The propafenone hydrochloride sustained-release capsule is prepared by filling sustained-release micro-tablets with the diameter of 2-3 mm, and has good dosage dispersion and uniformity. The micro tablets are composed of propafenone hydrochloride, a framework material, a lubricant and a diluent. The framework material is one or more of methyl cellulose, ethyl cellulose, hydroxypropyl methylcellulose and povidone; and a usage amount of the framework material is 1%-10%. The lubricant is one or more of sodium stearyl fumarate, magnesium stearate, micro-powder silica gel, talcum powder, calcium stearate and polyethylene glycol; and a usage amount of the lubricant is 0.1%-1.5%. The diluent is one or more of sucrose, mannitol, microcrystalline cellulose, lactose and polyethylene glycol; and a usage amount of the diluent is 2%-12%. According to the propafenone hydrochloride sustained-release capsule, the usage amount of a main drug is 77%-98%; and propafenone level in blood plasma reaches the maximum value 3-8 hours after drug delivery. The propafenone hydrochloride sustained-release capsule is stable in drug release and has a good sustained-release effect. An object of the invention is to provide a preparation method of the propafenone hydrochloride sustained-release capsule. The preparation method is simple in process, high in yield, good in stability and suitable for large-scale production.
Description
technical field [0001] The invention relates to a propafenone hydrochloride preparation, especially a slow-release preparation and a preparation method thereof. Background technique [0002] Propafenone hydrochloride (C 21 h 27 NO 3 HCl) is a class Ic antiarrhythmic drug with local anesthetic effect, and its main function is to block the sodium ion channel of cardiomyocytes. Clinically used for the treatment of ventricular, sinus, supraventricular tachycardia and pre-excitation syndrome. It was synthesized for the first time by German Hxlppharm company in 1975, and successfully imitated in my country in 1982. Commonly used oral preparations start to act 0.5 to 1 hour after taking them, reach the peak plasma concentration in 2 to 3 hours, and last for 4 to 8 hours. Therefore, the therapeutic dose of ordinary tablets is taken 3 to 6 times a day, which brings a lot of inconvenience. Moreover, the adverse reactions mostly appear at the peak time after administration, so the...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/52A61K31/138A61K47/38A61K47/36A61K47/32A61P9/06
Inventor 刘强宋远涛苏艺杰蔡林辉陈伟光朱海健叶英
Owner LP PHARM (XIAMEN) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com