Clopidogrel tablet and preparation method thereof
A technology of clopidogrel tablets and clopidogrel tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as instability of damp heat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
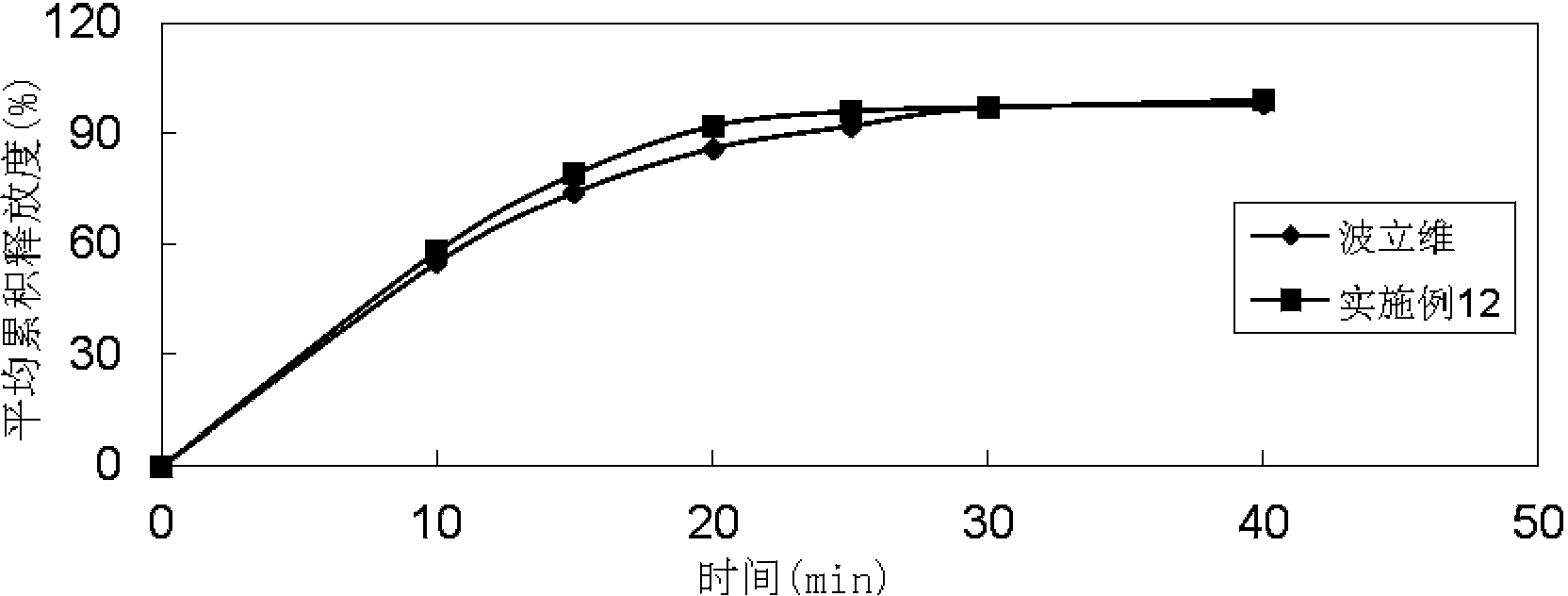
Embodiment 1
[0084]
[0085]Preparation method: (1) Clopidogrel hydrogen sulfate, sodium stearyl fumarate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone are pulverized separately, passed through a 30-mesh sieve, and set aside ; (2) Mix clopidogrel hydrogen sulfate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone evenly according to the prescription ratio, and set aside; (3) Stearin Sodium fumarate is added in the mixture of step (2), mix well; (4) measure the content of active ingredient in the mixed material, 8kg / mm 2 Press the tablet to get the plain tablet; (5) Coat the plain tablet obtained in step (4) with Opadry film, the coating temperature is 35°C-45°C, until the weight gain is 3% (the coating solution during the coating process It will be sprayed in the form of mist and equipped with a heating device, and the moisture in the coating solution will dry quickly. Therefore, the weight ga...
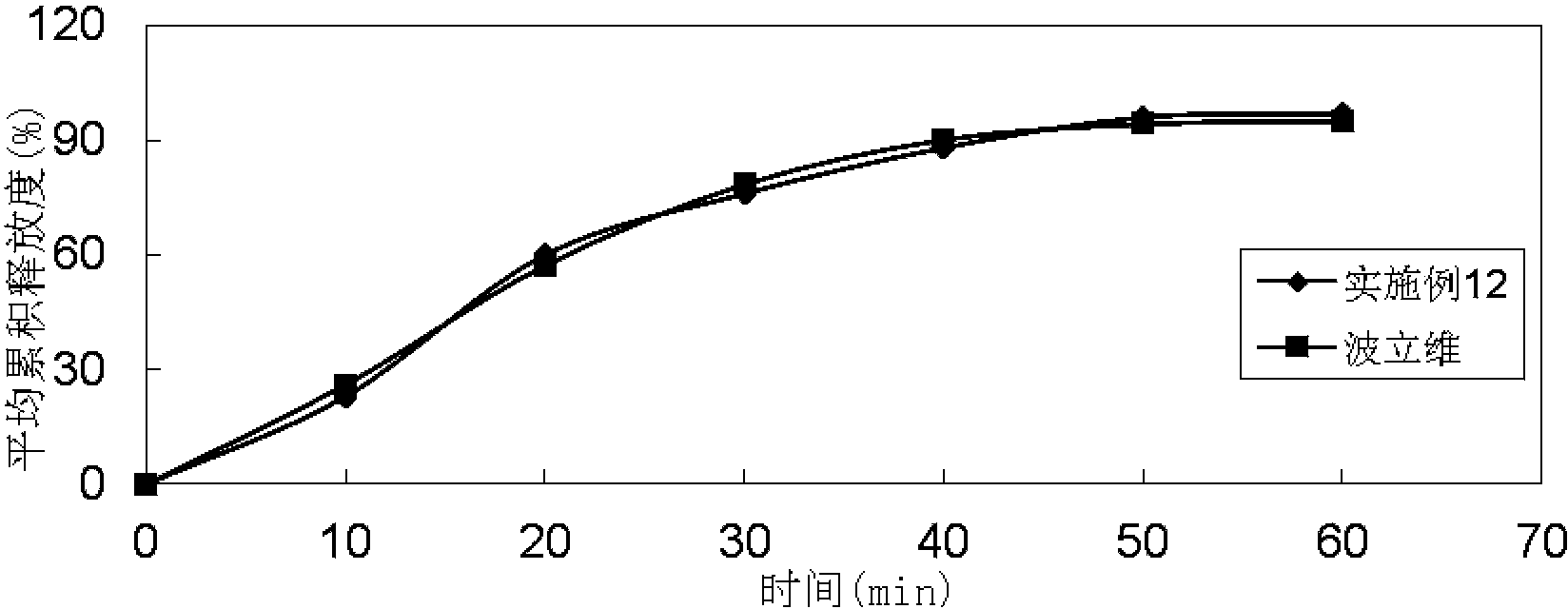
Embodiment 2
[0087]
[0088] Preparation method: (1) Clopidogrel hydrogen sulfate, sodium stearyl fumarate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone are pulverized separately, passed through a 30-mesh sieve, and set aside ; (2) Mix clopidogrel hydrogen sulfate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone evenly according to the prescription ratio, and set aside; (3) Stearin Sodium fumarate is added in the mixture of step (2), mix well; (4) measure the content of active ingredient in the mixed material, 8kg / mm 2 Press the tablet to obtain plain tablets; (5) Coat the plain tablets obtained in step (4) with Opadry film, and the coating temperature is 35°C to 45°C until the weight gain is 2.7%.
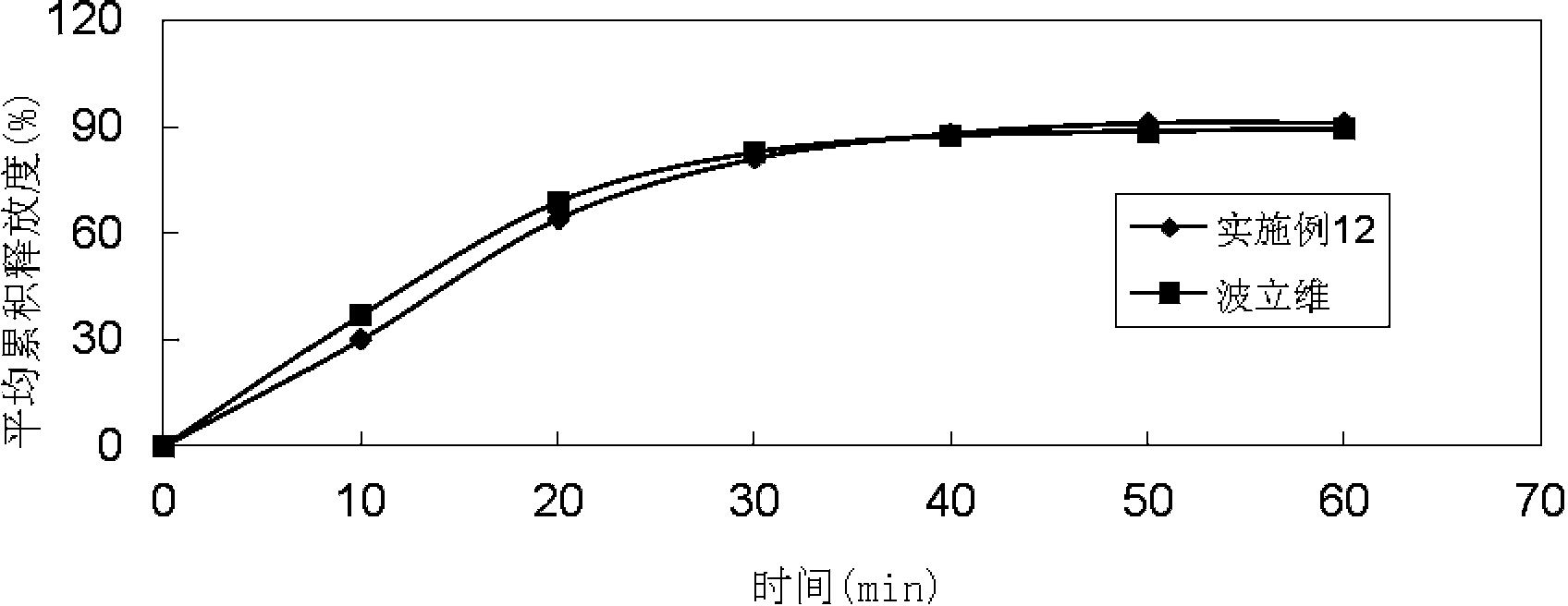
Embodiment 3
[0090]
[0091] Preparation method: (1) Clopidogrel hydrogen sulfate, sodium stearyl fumarate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone are pulverized separately, passed through a 30-mesh sieve, and set aside ; (2) Mix clopidogrel hydrogen sulfate, talcum powder, mannitol, microcrystalline cellulose, hydroxypropyl cellulose, and crospovidone evenly according to the prescription ratio, and set aside; (3) Stearin Sodium fumarate is added in the mixture of step (2), mix well; (4) measure the content of active ingredient in the mixed material, 8kg / mm 2 Press the tablet to obtain plain tablets; (5) Coat the plain tablets obtained in step (4) with Opadry film, and the coating temperature is 35°C to 45°C until the weight gain is 3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com